|

by Rady Ananda
January 6, 2012
from
ActivistPost Website
|
Rady Ananda is an
investigative reporter and researcher in the areas of
health, environment, politics, and civil liberties. Her
two websites, Food Freedom and COTO Report are essential
reading. |
The first ever lawsuit concerning risks
of nanotechnology was filed in federal court last month when several
groups jointly sued the US Food and Drug Administration (FDA)
for its lack of response to a 2006 petition demanding that products
with nanomaterials be labeled and their affects tested for safety.
Led by the International Center for
Technology Assessment (ICTA), plaintiffs also include,
'It is unacceptable that the FDA
continues to allow unregulated and unlabeled nanomaterials to be
used in products consumers use every day,' said Wenonah Hauter,
executive director of Food & Water Watch.
'It is past time for this agency to
live up to its mission and protect public health by assessing
the health and environmental risks of nanomaterials, and to
require labeling so that consumers know where these new
materials are being used.'
Based on the scientific literature so
far, several hundred products should be recalled due to their
toxicity to lab animals and bacteria.
Much of the
2011 complaint argues that because nanomaterials are
patented, and exhibit novel characteristics unique to their size,
they clearly represent new substances requiring regulation and
safety tests. Plaintiffs demand a recall on all such products until
their safety is proven.
Consumer groups including some of the same plaintiffs in the current
lawsuit also filed petitions urging nanotech regulation with the
Environmental Protection Agency back in 2006 and 2008, reports the
Chemical Regulation Reporter.
Nanotechnology is the science of manipulating materials on an atomic
or molecular scale, measured in billionths of a meter.
Nanotech-engineered materials (NEMs) are used in,
...with little regulation in the United States.
They
are found in ice cream and the coating sprayed on fruits and
vegetables, and even line bottles and cans, reported Andrew
Schneider in
his 2010 exposé on the subject.
NEMs are also used in industry processes and military applications
including drones, combat gear and miniature surveillance devices.
The Dept. of Defense has spent billions on nanotech R&D.
Per its
2007 report, nanotech is used in,
“chemical and biological warfare
defense; high performance materials for platforms and weapons;
unprecedented information technology [like smart clothes];
revolutionary energy and energetic materials; and uninhabited
vehicles and miniature satellites.”
(Also see the
2011 National
Nanotechnology Initiative Strategic Plan.)
In June 2011, both
the FDA and
the EPA
issued draft guidelines on NEMs.
Though nano-pesticides are
already on
the market, the EPA made its
first approval last month. The Swiss
firm, HeiQ, now sells its composite nanosilver and nano-silica for
use in clothing (to reduce microbial odor) with EPA approval.
Upon publication of the FDA’s voluntary guidelines, the
Alliance for
Natural Health immediately demanded that nanomaterials be banned
from organic certification, as they are in Canada.
The FDA has done nothing on NEM regulation since last June. Prior to
that, the FDA absurdly denied that any nanofoods were being sold in
the U.S.
“Not true, say some of the agency’s
own safety experts, pointing to scientific studies published in
food science journals, reports from foreign safety agencies and
discussions in gatherings like the Institute of Food
Technologists conference,” reports Schneider.
Several of the plaintiffs have issued
public reports on nanotech, including IATP.
In
Racing Ahead: U.S. Agri-Nanotechnology
in the Absence of Regulation, IATP notes that as of March 2011,
there were over 1,300 products known to contain NEMs. That’s up from
200 in 2006, but the number is conservatively expected to rise to
3,400 by 2020.
The ETC Group estimates well over 1,600 products in
its 2010 report,
The Big Downturn? Nanogeopolitics.
The Project on Emerging Nanotechnologies (PEN), a partnership of the
Pew Charitable Trusts and the Woodrow Wilson International Center
for Scholars, a U.S. government research center, notes that there is
no registry of nano-scale ingredients and materials used in products
or industrial processes.
“Establishing such a registry, as
well as consumer products registry,” advises IATP, “would be
necessary components of the eventual regulation of
nanotechnology.”
Meanwhile, some products containing
nanomaterials can be found with
PEN’s iPhone application for using
the Nanotechnology Products Inventory.
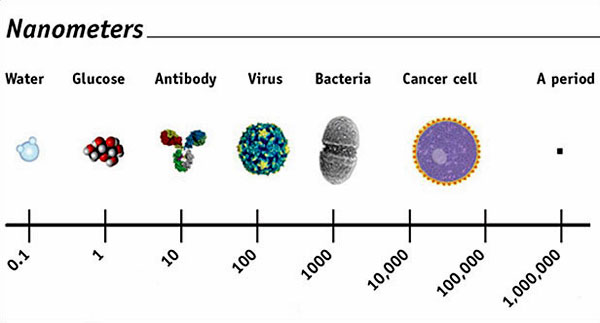
Size Matters
“There is a dependent relationship
between size and surface area and nanoparticle toxicity; as
particles are engineered smaller on the nano-level, they are
more likely to be toxic,” plaintiffs wrote in their
2006
petition.
“Many relatively inert and stable
chemicals, such as carbon, pose toxic risk in their nano-scale
form.”
That small of a size makes nanoparticles
capable of crossing the blood-brain barrier noted food research
scientist Ellin Doyle.
In 2006,
she published a literature
review on nanotechnology advising,
“Nanoparticles are readily taken up
by many types of cells in vitro and are expected to cross the
blood-brain barrier that excludes many substances that might
harm the brain.”
Despite this, in 2006, the FDA ruled
that “particle size is not an issue” for regulation.
Basic chemistry teaches the opposite. At
that small of a scale, a particle’s electrochemical features more
heavily influence its interactions with nearby substances, including
viruses, bacteria and DNA.
The small nanosize means there are more
atoms on its surface than inside the particle.
Both the US Patent Office and the National Nanotechnology
Initiative, an agency of the US National Science Foundation, refuted
the FDA’s stance, explaining that the small size of nanoparticles
enable “unique and novel characteristics” that impact not only
electrochemical interactions, but also their optical, photoreactive,
magnetic, persistence, bio-accumulation, toxicity and explosiveness
features.
Nanosized aluminum, a suspected
ingredient in chemtrails, has been
shown to spontaneously combust, reports ETC. Texas-based Quantum
Logic Devices holds
Patent No. 7,338,711, which is an “enhanced nanocomposite combustion accelerant” used in fuels, propellants and
explosives.
In its
2007 Nanotechnology Task Force Report, the FDA finally
reversed itself admitting that,
“at this scale, properties of a
material relevant to the safety and (as applicable)
effectiveness of FDA-regulated products might change repeatedly
as size enters into or varies within the nanoscale range.”
Nanohazards
In addition to several studies showing nanosize-induced harm cited
in the 2006 petition, ETC listed
ten studies from 1997 through early
2004 that showed DNA and brain damage, lung dysfunction, and
bioaccumulation (whereby earthworms and other creatures absorb,
inhale or ingest the nanoparticles and pass them up the food chain).
This is especially significant as nanopollution grows with the
release of thousands of pounds of nanomaterials into the
environment, notes Friends of the Earth in its 2006 report,
Nanomaterials, sunscreens and cosmetics. (More studies can be found
at this
companion FOE report.)
ETC also pointed to studies showing that nanoparticles can break
down in the body causing metal poisoning, and can cross the placenta
from mother to unborn fetus.
A
2010 British study confirmed that anything smaller than 100 nm
poses even greater health risks because it can “access all areas of
the body” and can even penetrate the nucleus of cells where DNA is
located.
Stronger than steel, carbon nanotubes
look and act like asbestos,
which causes lung cancer. This
FOE report also cites reduced kidney
growth in lab animals exposed to nanomaterials.
Under its 2011 guidelines, the FDA will consider particles that are
from 1 to 100 nanometers in size, but up to one micron if the end
products,
“exhibit properties or phenomena,
including physical or chemical properties or biological effects,
that are attributable to its dimension(s).”
By dragging its feet on nanotech
regulation for the past several years, the FDA and EPA have allowed
the proliferation of nanomaterials into consumer products without
disclosure.
Similar to the federal government’s
refusal to label genetically modified foods, US consumers are once
again lab animals for the biotech industry.
Hopefully, this lawsuit will spur
appropriate safety testing and the removal of unsafe products from
the market.
|


