|

by Andrew Schneider
Andrew Schneider Senior Public Health
Correspondent
March 24, 2010
from
AOLNews Website
First in a Three-Part Series
Amid Nanotech's Dazzling Promise, Health Risks
Grow
For almost two years, molecular
biologist Bénédicte Trouiller doused the drinking water of
scores of lab mice with nano-titanium dioxide, the most common
nanomaterial used in consumer products today.
She knew that earlier studies conducted in test tubes and petri
dishes had shown the same particle could cause disease. But her
tests at a lab at UCLA's School of Public Health were in vivo -
conducted in living organisms - and thus regarded by some scientists
as more relevant in assessing potential human harm.
Halfway through, Trouiller became alarmed: Consuming the nano-titanium
dioxide was damaging or destroying the animals' DNA and chromosomes.
The biological havoc continued as she repeated the studies again and
again.
It was a significant finding: The
degrees of DNA damage and genetic instability that the 32-year-old
investigator documented can be,
"linked to all the big killers of
man, namely cancer, heart disease, neurological disease and
aging," says Professor Robert Schiestl, a genetic toxicologist
who ran the lab at UCLA's School of Public Health where
Trouiller did her research.

Benedicte Trouiller
in an undated photo
Courtesy Benedicte Trouiller
UCLA
molecular biologist Bénédicte Trouiller found
that nano-titanium dioxide - the nanomaterial
most commonly used in consumer products today -
can damage or destroy DNA and chromosomes at
degrees that can be linked to "all the big
killers of man," a colleague says.
Nano-titanium dioxide is so pervasive
that the Environmental Working Group says it has calculated that
close to 10,000 over-the-counter products use it in one form or
another.
Other public health specialists put the
number even higher.
It's "in everything from medicine capsules and
nutritional supplements, to food icing and additives, to skin
creams, oils and toothpaste," Schiestl says.
He adds that at least 2
million pounds of nanosized titanium dioxide are produced and used
in the U.S. each year.
What's more, the particles Trouiller gave the mice to drink are just
one of an endless number of engineered, atom-size structures that
have been or can be made. And a number of those nanomaterials have
also been shown in published, peer-reviewed studies (more than 170
from the National Institute for Occupational Safety and Health
alone) to potentially cause harm as well.
Researchers have found, for instance,
that
carbon nanotubes - widely used in many industrial applications
- can penetrate the lungs more deeply than asbestos and appear to
cause asbestos-like, often-fatal damage more rapidly.
Other nanoparticles, especially those
composed of metal-chemical combinations, can cause cancer and birth
defects; lead to harmful buildups in the circulatory system; and
damage the heart, liver and other organs of lab animals.
Yet despite those findings, most federal agencies are doing little
to nothing to ensure public safety. Consumers have virtually no way
of knowing whether the products they purchase contain nanomaterials,
as under current U.S. laws it is completely up to manufacturers what
to put on their labels.
And hundreds of interviews conducted by
AOL News' senior public health correspondent over the past 15 months
make it clear that movement in the government's efforts to institute
safety rules and regulations for use of nanomaterials is often as
flat as the read-out on a snowman's heart monitor.
"How long should the public have to
wait before the government takes protective action?" says Jaydee
Hanson, senior policy analyst for the Center for Food Safety.
"Must the bodies stack up first?"
Big Promise
Comes With Potential Perils
"Nano" comes from the Greek word for dwarf, though that falls short
of conveying the true scale of this new world: Draw a line 1 inch
long, and 25 million nanoparticles can fit between its beginning and
end.
Apart from the materials' size, everything about nanotechnology is
huge. According to the federal government and investment analysts,
more than 1,300 U.S. businesses and universities are involved in
related research and development.
The National Science Foundation says
that $60 billion to $70 billion of nano-containing products are sold
in this country annually, with the majority going to the energy and
electronics industries.
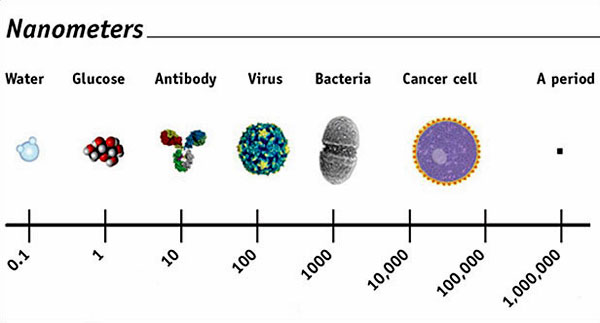
Both the
promise and the potential peril of nanomaterials
come from their staggeringly small size, which is
highlighted by the chart above.
(Note, for
example, how it shows that the periods on this page
are equal to 1 million nanometers.)
Despite the speed bump of the recession,
a global market for nano-containing products that stood at $254
billion in 2005 is projected to grow to $2.5 trillion over the next
four years, says Michael Holman, research director of Boston-based Lux Research.
Another projection, this one from
National Science Foundation senior nanotechnology adviser Mihail
Roco, says that nanotech will create at least 1 million jobs
worldwide by 2015.
By deconstructing and then reassembling atoms into previously
unknown material - the delicate process at the heart of
nanotechnology - scientists have achieved medical advancements that
even staunch critics admit are miraculous. Think of a medical smart
bomb: payloads of cancer-fighting drugs loaded into nanoscale
delivery systems and targeted against a specific tumor.
Carbon nanotubes, rod-shaped and rigid with a strength that
surpasses steel at a mere fraction of the weight, were touted by
commentators at the Vancouver Olympics as helmets, skis and bobsleds
made from nanocomposites flashed by. Those innovations follow
ultralight bicycles used in the Tour de France, longer-lasting
tennis balls, and golf balls touted to fly straighter and roll
farther.
Food scientists, meanwhile, are almost gleeful over the ability to
create nanostructures that can enhance food's flavor, shelf life and
appearance - and to one day potentially use the engineered particles
to craft food without ever involving a farm or ranch.
Yet for all the technology's promise and relentless progress, major
questions remain about nanomaterials' effects on human health.
A
bumper sticker spotted near the sprawling Food and Drug
Administration complex in Rockville, Md., puts it well:
"Nanotech -
wondrous, horrendous, and unknown."
Adds Jim Alwood, nanotechnology coordinator in the
Environmental Protection Agency's Office of Pollution Prevention and
Toxics:
"There is so much uncertainty about
the questions of safety. We can't tell you how safe or unsafe
nanomaterials are. There is just too much that we don't yet
know."
What is known is by turns fascinating
and sobering.

Vial of carbon nanotubes
The
carbon nanotubes in this vial are part of a
booming industry. According to one consulting
firm, the global market for nano-containing
products is projected to grow to $2.5 trillion
by 2014.
Nanoparticles can heal, but they can
also kill.
Thanks to their size, researchers have
found, they can enter the body by almost every pathway. They can be
inhaled, ingested, absorbed through skin and eyes. They can invade
the brain through the olfactory nerves in the nose.
After penetrating the body, nanoparticles can enter cells, move from
organ to organ and even cross the protective blood-brain barrier.
They can also get into the bloodstream, bone marrow, nerves,
ovaries, muscles and lymph nodes.
The toxicity of a specific nanoparticle depends, in part, on its
shape and chemical composition. Many are shaped roughly like a
soccer ball, with multiple panels that can increase reactivity, thus
exacerbating their potential hazards.
Some nanoparticles can cause a condition called oxidative stress,
which can inflame and eventually kill cells.
A potential blessing in controlled
clinical applications, this ability also carries potentially
disastrous consequences.
"Scientists have engineered
nanoparticles to target some types of cancer cells, and this is
truly wonderful," says Dr. Michael Harbut, director of the
Environmental Cancer Initiative at Michigan's Karmanos Cancer
Institute.
"But until we have sufficient
knowledge of, and experience with, this 21st-century version of
the surgical scalpel, we run a very real risk of simultaneously
destroying healthy cells."
When incorporated into food products,
nanomaterials raise other troubling vagaries.
In a report issued in
January, the science committee of the British House of Lords,
following a lengthy review, concluded that there was too little
research looking at the toxicological impact of eating nanomaterials.
The committee recommended that such
"products will simply be denied regulatory approval until further
information is available," and also raised the concern that while
the amount of nanomaterial in food may be small, the particles can
accumulate from repeated consumption.
"It is chronic exposure to nanomaterials that is arguably more
relevant to food science applications," says Bernadene Magnuson, a
food scientist and toxicologist with Cantox Health Sciences
International.
"Prolonged exposure studies must be
conducted."
Given the potential hazards, public
health advocates are calling for greater restraint on the part of
those rushing nano-products to market.
"The danger is there today in the
hundreds of nano-containing consumer products being sold," says
Jennifer Sass, senior scientist and nano expert for the
nonpartisan Natural Resources Defense Council.
"Things that are in the nanoscale
that are intentionally designed to be put into consumer products
should be instantly required to be tested, and until proper risk
assessments are done, they shouldn't be allowed to be sold."
David Hobson, chief scientific officer
for international risk assessment firm nanoTox, adds that the
questions raised by the growing body of research,
"are significant enough that we
should begin to be concerned. We should not wait until we see
visible health effects in humans before we take steps to protect
ourselves or to redesign these particles so that they're safer."
Hobson says that when he talks to
university and industry nano scientists, he sometimes feels as if
he's talking with Marie Curie when she first was playing around with
radium.
"It's an exciting advancement
they're working with," he says. "But no one even thinks that it
could be harmful."
More on Why Size
Matters
At a weeklong Knight Foundation Science Workshop on nanotechnology
at the Massachusetts Institute of Technology in June, five
professors - four from the Cambridge school and one from Cornell
University - dazzled their fellow participants with extensive
show-and-tells on the amazing innovations coming out of their labs.
At one point, one played a video of a mouse with a severed spine
dragging his lifeless rear legs around his cage.
A scaffolding made
of nanomaterial was later implanted across the mouse's injury.
Further footage showed the same rodent, 100 days later, racing
around his enclosure, all four legs churning like mad.
When the five nanotech pioneers were asked about hazards from the
particles they were creating, only one said she was watching new
health studies closely. The others said size had no impact on risk:
No problems were expected, since the same chemicals they had
nano-ized had been used safely for years.
It's an argument echoed by researchers and nano-manufacturers around
the globe. But those assumptions are challenged by the many research
efforts presenting strong evidence to the contrary, among them
Trouiller's study, which was published in November.
"The difference in size is vital to
understanding the risk from the same chemical," says Schiestl,
who was a co-author on the UCLA study.
"Titanium dioxide is chemically
inert and has been safely used in the body for decades for joint
replacements and other surgical applications. But when the very
same chemical is nanosized, it can cause illness and lead to
death."
Regulators Take a
'Wait-and-See' Approach
Many public health groups and environmental activists fear the
government's lethargy on nanotechnology will be a repeat of earlier
regulatory snafus where deadly errors were made in assessing the
risk of new substances.
"The unsettling track record of
other technological breakthroughs - like asbestos, DDT, PCBs and
radiation - should give regulators pause as they consider the
growing commercial presence of nanotech products," says Patty
Lovera, assistant director of Food & Water Watch.
"This wait-and-see approach puts
consumers and the environment at risk."
While the agency has many critics, the
EPA, for its part, is pursuing an aggressive strategy on
nanotechnology.
Among nano-titanium dioxide's other
uses, the particle is deployed as an agent for removing arsenic from
drinking water, and last year, the EPA handed out 500-page books of
health studies on the particles to a panel of scientists asked to
advise the agency on the possible risk of that practice.
(Another EPA science advisory board held
hearings into the hazards from nanosilver used in hundreds of
products, from pants, socks and underwear to teething rings.)

Dr.
Jesse Goodman, the FDA's chief scientist and
deputy commissioner for science and public
health, says that "there is a most definite
requirement that manufacturers ensure that
the products be safe." But he adds that
compliance is essentially voluntary. The FDA
takes action only after an unsafe product is
reported.
The Food and Drug Administration's
(FDA) handling of nano-titanium dioxide provides a more emblematic example
of the government's overall approach.
Public health advocates and some of the
FDA's own risk assessors are frustrated by what they perceive as the
agency's "don't look, don't tell" philosophy. The FDA doesn't even
make a pretense of evaluating nanoparticles in the thousands of
cosmetics, facial products or food supplements that have already
flooded the market, even those that boast the presence of engineered
particles.
Nano Gold Energizing Cream ($420 a jar)
and Cyclic nano-cleanser ($80 a bar) are among the many similar
products unevaluated by the agency.
Dr. Jesse Goodman, the FDA's chief scientist and deputy
commissioner for science and public health, says the exclusion of
cosmetics and nutritional supplements from its regulations is what
Congress wants.
Goodman adds that,
"there is a most definite
requirement that manufacturers ensure that the products be
safe",
...but says that compliance is
essentially voluntary, with the FDA taking action only after an
unsafe product is reported.
AOL News repeatedly asked what steps the FDA was taking regarding
nano-titanium dioxide, whose risks are acknowledged by other
regulatory bodies, including the EPA and the NIOSH.
The slow-to-arrive answer from
spokeswoman Rita Chappelle:
"If information were to indicate
that additional safety evaluation or other regulatory action is
warranted, we would work with all parties to take the steps
appropriate to ensure the safety of marketed products."
Chappelle says FDA scientists are
conducting research that focuses on nano-titanium dioxide, but
declines to offer any details.
Several of the agency's own safety
experts say they specifically have urged that the engineered
structures not be used in any products they do regulate without
appropriate safety testing.
Why Nano-Optimists
Hold the Upper Hand
Many government investigators join civilian public health
specialists in denouncing the scant money that goes to exploring
nanomaterials' possibly wicked side effects.
The 2011 federal budget proposes
spending $1.8 billion on nanotechnology, but just $117 million, or
6.6 percent, of that total was earmarked for the study of safety
issues.
The Obama administration says it is being appropriately vigilant
about nanotech.
"This administration takes
nanotechnology-related environment, health and safety very
seriously. It is a significant priority," says Travis Earles,
assistant director for nanotechnology in the White House Office
of Science and Technology Policy.
After taking office, he adds,
"We were able to immediately
increase the spending in those areas."
But Earles, in what has become standard
federal practice, is more fixated on nanotech's upsides.
"We are talking about new jobs, new
markets, economic and societal benefits so broad they stretch
the imagination," he says. Yes, "absolutely," there are reasons
for caution, he says.
"But you can't refer to
nanotechnology as a monolithic entity. Risk assessment depends
fundamentally on context - it depends on the specific
application and the specific material."
There's some scientific basis for this
emphasize-the-positive position.
"Every time you find a hazardous
response in a test tube, that should not necessarily be
construed as a guarantee of a real-life adverse outcome," notes
Dr. Andre Nel, chief of the division of nanomedicine at the
California Nanosystems Institute at UCLA.
But there are two ways to proceed in the
face of such uncertainty.
One is to forge ahead, assuming the best
- that this will be one of those times where the lab results don't
correlate to real-world experiences. Another is to hit pause and do
the additional testing necessary to be sure that sickened lab
animals do not portend human harm.
For advocates of more precautions for nanotech, the latter is the
only responsible course.
"From cosmetics to cookware to food,
nanoparticles are making their way into every facet of consumer
life with little to no oversight from government regulators,"
says Lovera from Food & Water Watch.
"There are too many unanswered
questions and common-sense demands that these products be kept
off the market until their safety is assured."
With a moratorium not a realistic
option, the U.S. government, along with its counterparts abroad, is
left to tread gingerly in responding to the emerging evidence of
nanotechnology's potential hazards.
"They don't want to cause either a
collapse in the industry or generate any kind of public backlash
of any sort," says Pat Mooney, executive director of ETC Group,
an international safety and environmental watchdog.
"So they're in the background
talking about how they're going to tweak regulations - where in
fact a lot more than tweaking is required.
"They've got literally thousands of [nano] products in the
marketplace, and they don't have any safety regulations in
place," Mooney continues.
"These are things that we're rubbing
in our skin, spraying in our fields, eating and wearing. And
that's a mistake, and they're trying to figure out what to do
about it all."
Second in a Three-Part
Series
Regulated or Not, Nano-Foods Coming to A Store
Near You
For centuries, it was the cook and the
heat of the fire that cajoled taste, texture, flavor and aroma from
the pot.
Today, that culinary voodoo is being
crafted by white-coated scientists toiling in pristine labs,
rearranging atoms into chemical particles never before seen.
At last year's Institute of Food Technologists international
conference, nanotechnology was the topic that generated the most
buzz among the 14,000 food-scientists, chefs and manufacturers
crammed into an Anaheim, Calif., hall. Though it's a word that has
probably never been printed on any menu, and probably never will,
there was so much interest in the potential uses of nanotechnology
for food that a separate daylong session focused just on that
subject was packed to overflowing.
In one corner of the convention center, a chemist, a flavorist and
two food-marketing specialists clustered around a large chart of the
Periodic Table of Elements (think back to high school science
class).
The food chemist, from China, ran her
hands over the chart, pausing at different chemicals just long
enough to say how a nano-ized version of each would improve existing
flavors or create new ones.
One of the marketing guys questioned what would happen if the
consumer found out.
The flavorist asked whether the Food and Drug Administration would
even allow nanoingredients.
Posed a variation of the latter question, Dr. Jesse Goodman, the
agency's chief scientist and deputy commissioner for science and
public health, gave a revealing answer. He said he wasn't involved
enough with how the FDA was handling nanomaterials in food to
discuss that issue. And the agency wouldn't provide anyone else to
talk about it.
This despite the fact that hundreds of peer-reviewed studies have
shown that nanoparticles pose potential risks to human health - and,
more specifically, that when ingested can cause DNA damage that can
prefigure cancer and heart and brain disease.
Despite
Denials, Nano-Food Is Here
Officially, the FDA says there aren't any nano-containing food
products currently sold in the U.S.
Not true, say some of the agency's own safety experts, pointing to
scientific studies published in food science journals, reports from
foreign safety agencies and discussions in gatherings like the
Institute of Food Technologists conference.
In fact, the arrival of nanomaterial onto the food scene is already
causing some big-chain safety managers to demand greater scrutiny of
what they're being offered, especially with imported food and
beverages.
At a conference in Seattle last year
hosted by leading food safety attorney Bill Marler,
presenters raised the issue of how hard it is for large supermarket
companies to know precisely what they are purchasing, especially
with nanomaterials, because of the volume and variety they deal in.

According to a USDA scientist, some Latin
American packers spray U.S.-bound produce
with a wax-like nanocoating to extend
shelf-life.
"We found no indication that the nanocoating... has ever been tested for
health effects," the researcher says.
Craig Wilson, assistant vice
president for safety for
Costco, says his chain does not test for nanomaterial in the food products it is offered by manufacturers.
But, he adds, Costco is looking,
"far more carefully at everything we
buy... We have to rely on the accuracy of the labels and the
integrity of our vendors. Our buyers know that if they find
nanomaterial or anything else they might consider unsafe, the
vendors either remove it, or we don't buy it."
Another government scientist says
nanoparticles can be found today in produce sections in some large
grocery chains and vegetable wholesalers.
This scientist, a researcher with the
USDA's Agricultural Research Service, was part of a group that
examined Central and South American farms and packers that ship
fruits and vegetables into the U.S. and Canada.
According to the USDA researcher - who
asked that his name not be used because he's not authorized to speak
for the agency - apples, pears, peppers, cucumbers and other fruit
and vegetables are being coated with a thin, wax-like nanocoating to
extend shelf-life.
The edible nanomaterial skin will also
protect the color and flavor of the fruit longer.
"We found no indication that the
nanocoating, which is manufactured in Asia, has ever been tested
for health effects," said the researcher.

A science
committee of the British House of Lords has
found that nanomaterials are already appearing
in numerous products, among them salad dressings
and sauces.
Jaydee Hanson, policy analyst for
the Center for Food Safety, says that they're
also being added to ice cream to make it "look
richer and better textured."
Some foreign governments, apparently
more worried about the influx of nano-related products to their
grocery shelves, are gathering their own research. In January, a
science committee of the British House of Lords issued a lengthy
study on nanotechnology and food.
Scores of scientific groups and consumer
activists and even several international food manufactures told the
committee investigators that engineered particles were already being
sold in,
...to which they're added to ensure easy
pouring.
Other researchers responding to the committee's request for
information talked about hundreds more items that could be in stores
by year's end.
For example, a team in Munich has used nano-nonstick coatings to end
the worldwide frustration of having to endlessly shake an upturned
mustard or ketchup bottle to get at the last bit clinging to the
bottom.
Another person told the investigators
that Nestlé and Unilever have about completed developing a nano-emulsion-based
ice cream that has a lower fat content but retains its texture and
flavor.
The Ultimate
Secret Ingredient
Nearly 20 of the world's largest food manufacturers, among them,
-
Nestlé
-
Hershey
-
Cargill
-
Campbell Soup
-
Sara Lee
-
H.J. Heinz,
...have their own in-house nano-labs, or
have contracted with major universities to do nano-related food
product development.
But they are not eager to broadcast
those efforts.
[UPDATE: Campbell's
spokesman Anthony Sanzio says his company does not have a
nanotechnology program, but adds "We would be irresponsible to
ignore it."]
A team
in Munich, the House of Lords investigators
also learned, is using nano-nonstick
coatings to make it easier to get the last
drops of ketchup out of the bottle.
Kraft was the first major food company
to hoist the banner of nanotechnology.
Spokesman Richard Buino, however,
now says that while,
"we have sponsored nanotech research
at various universities and research institutions in the past,"
Kraft has no labs focusing on it today.
The stance is in stark contrast to the
one Kraft struck in late 2000, when it loudly and repeatedly
proclaimed that it had formed the Nanotek Consortium with engineers,
molecular chemists and physicists from 15 universities in the U.S.
and abroad.
The mission of the team was to show how
nanotechnology would completely revolutionize the food manufacturing
industry, or so said its then-director, Kraft research chemist
Manuel Marquez.
But by the end of 2004, the much-touted operation seemed to vanish.
All mentions of Nanotek Consortium
disappeared from Kraft's news releases and corporate reports.
"We have not nor are we currently
using nanotechnology in our products or packaging," Buino added
in another e-mail.
Industry
Tactics Thwart Risk Awareness
The British government investigation into nanofood strongly
criticized the U.K.'s food industry for,
"failing to be transparent about its
research into the uses of nanotechnologies and nanomaterials."
On this side of the Atlantic, corporate
secrecy isn't a problem, as some FDA officials tell it.
Investigators on Capitol Hill say the FDA's congressional liaisons
have repeatedly assured them - from George W. Bush's administration
through President Barack Obama's first year - that the big U.S. food
companies have been upfront and open about their plans and progress
in using nanomaterial in food.
But FDA and USDA food safety specialists interviewed over the past
three months stressed that based on past performance, industry
cannot be relied on to voluntarily advance safety efforts.
These government scientists, who are actively attempting to evaluate
the risk of introducing nanotechnology to food, say that only a
handful of corporations are candid about what they're doing and
collaborating with the FDA and USDA to help develop regulations that
will both protect the public and permit their products to reach
market.
Most companies, the government
scientists add, submit little or no information unless forced.
Even then, much of the information
crucial to evaluating hazards - such as the chemicals used and
results of company health studies - is withheld, with corporate
lawyers claiming it constitutes confidential business information.
Both regulators and some industry consultants say the evasiveness
from food manufacturers could blow up in their faces.
As precedent, they point to what
happened in the mid-'90s with genetically modified food, the last
major scientific innovation that was, in many cases, force-fed to
consumers.
"There was a lack of transparency on
what companies were doing. So promoting genetically modified
foods was perceived by some of the public as being just
profit-driven," says Professor Rickey Yada of the Department of
Food Science at the University of Guelph in Ontario, Canada.
"In retrospect, food manufacturers
should have highlighted the benefits that the technology could bring
as well as discussing the potential concerns."
Eating
Nanomaterials Could Increase Underlying Risks
The House of Lords' study identified "severe shortfalls" in research
into the dangers of nanotechnology in food.
Its authors called for funding studies
that address the behavior of nanomaterials within the digestive
system.
Similar recommendations are being made
in the U.S., where the majority of research on nanomaterial focuses
on it entering the body via inhalation and absorption.
The food industry is very competitive, with thin profit margins.
And safety evaluations are very
expensive, notes Bernadene Magnuson, senior scientific and
regulatory consultant with risk-assessment firm Cantox Health
Sciences International.
"You need to be pretty sure you've
got something that's likely to benefit you and your product in
some way before you're going to start launching into safety
evaluations," she explains.
Magnuson believes that additional
studies must be done on chronic exposure to and ingestion of
nanomaterials.
One of the few ingestion studies recently completed was a
two-year-long examination of nano-titanium dioxide at UCLA, which
showed that the compound caused DNA and chromosome damage after lab
animals drank large quantities of the particles in their water.

Meat cooler at grocery store
Sono-Tek, a company based in Milton, N.Y.,
employs nanotechnology in its industrial
sprayers.
"One new application for us is
spraying nanomaterial suspensions onto
biodegradable plastic food wrapping
materials to preserve the freshness of food
products," says its chairman and CEO.
It is widely known that nano-titanium
dioxide is used as filler in hundreds of medicines and cosmetics and
as a blocking agent in sunscreens.
But Jaydee Hanson, policy analyst
for the Center for Food Safety, worries that the danger is greater,
"when the nano-titanium dioxide is
used in food."
Ice cream companies, Hanson says, are
using nanomaterials to make their products "look richer and better
textured."
Bread makers are spraying nanomaterials
on their loaves,
"to make them shinier and help them
keep microbe-free longer."
While AOL News was unable to identify a
company pursuing the latter practice, it did find Sono-Tek of
Milton, N.Y., which uses nanotechnology in its industrial sprayers.
"One new application for us is
spraying nanomaterial suspensions onto biodegradable plastic
food wrapping materials to preserve the freshness of food
products," says Christopher Coccio, chairman and CEO.
He said the development of this nano-wrap
was partially funded by New York State's Energy Research and
Development Authority.
"This is happening," Hanson says.
He calls on the FDA to,
"immediately seek a ban on any
products that contain these nanoparticles, especially those in
products that are likely to be ingested by children."
"The UCLA study means we need to research the health effects of
these products before people get sick, not after," Hanson says.
There is nothing to mandate that such
safety research take place.
The FDA's
Blind Spot
The FDA includes titanium dioxide among the food additives it
classifies under the designation "generally recognized as safe," or
GRAS.
New additives with that label can bypass
extensive and costly health testing that is otherwise required of
items bound for grocery shelves.
A report issued last month by the Government Accountability Office
(GAO) denounced the enormous loophole that the FDA has permitted through
the GRAS classification. And the GAO investigators also echoed the
concerns of consumer and food safety activists who argue that giving nanomaterials the GRAS free pass is perilous.
Food safety agencies in Canada and the European Union require all
ingredients that incorporate engineered nanomaterials to be
submitted to regulators before they can be put on the market, the
GAO noted.
No so with the FDA.
"Because GRAS notification is
voluntary and companies are not required to identify
nanomaterials in their GRAS substances, FDA has no way of
knowing the full extent to which engineered nanomaterials have
entered the U.S. food supply," the GAO told Congress.
Amid that uncertainty, calls for safety
analysis are growing.
"Testing must always be done," says
food regulatory consultant George Burdock, a toxicologist and
the head of the Burdock Group. "Because if it's nanosized, its
chemical properties will most assuredly be different and so
might the biological impact."
Will Consumers
Swallow What Science Serves Up Next?
Interviews with more than a dozen food scientists revealed
strikingly similar predictions on how the food industry will employ
nanoscale technology.
They say firms are creating
nanostructures to enhance flavor, shelf life and appearance. They
even foresee using encapsulated or engineered nanoscale particles to
create foods from scratch.
Experts agreed that the first widespread use of nanotechnology to
hit the U.S. food market would be nanoscale packing materials and
nanosensors for food safety, bacteria detection and traceability.
While acknowledging that many more nano-related food products are on
the way, Magnuson, the industry risk consultant, says the greatest
degree of research right now is directed at food safety and quality.
"Using nanotechnology to improve the
sensitivity and speed of detection of food-borne pathogens in
the food itself or in the supply chain or in the processing
equipment could be lifesaving," she says.
For example, researchers at Clemson
University, according to USDA, have used nanoparticles to identify
campylobacter, a sometimes-lethal food-borne pathogen, in poultry
intestinal tracts prior to processing.
At the University of Massachusetts Amherst, food scientist Julian
McClements and his colleagues have developed time-release
nanolaminated coatings to add bioactive components to food to
enhance delivery of ingredients to help prevent diseases such as
cancer, osteoporosis, heart disease and hypertension.
But if the medical benefits of such an application are something to
cheer, the prospect of eating them in the first place isn't viewed
as enthusiastically.
Advertising and marketing consultants for food and beverage makers
are still apprehensive about a study done two years ago by the
German Federal Institute of Risk Assessment, which commissioned
pollsters to measure public acceptance of nanomaterials in food.
The study showed that only 20 percent of
respondents would buy nanotechnology-enhanced food products.
Third in a Three-Part Series
Obsession With Nanotech Growth Stymies
Regulators
When the United States government
formally acknowledged the world-changing potential of nanotechnology
a decade ago, it was decided that America should lead the way.
Almost immediately, 25 different federal
agencies began scrambling to find uses for the engineered particles
in medicine, energy, transport, weapons, protective devices and
food, as well as thousands more real and dreamed-about applications.
Today, the U.S. is at the fore of worldwide nano-innovation.
But when it comes to regulations and
laws that will protect consumers and workers from the potential
hazards, the country lags badly behind many other nations.
"The government agencies responsible
for protecting the public from the adverse effects of these
technologies seem worn and tattered," former Environmental
Protection Agency assistant administrator Clarence Davies wrote
in a study for the Woodrow Wilson International Center for
Scholars' Project on Emerging Nanotechnologies, where he is now
a senior adviser.
Davies, who while at the EPA authored
what became its all-important Toxic Substances Control Act, adds
that the gap between the capabilities of nanotechnology and those of
the regulatory system,
"is likely to widen as the new
technologies advance."

Andrew Maynard in an undated photo
Andrew Maynard, chief science adviser for the
Woodrow Wilson International Center for
Scholars, is among those advocating guidelines
for nano-safety.
"Get these rules wrong - and
we're not sure what they are yet - or ignore
them, and we may cause unnecessary harm to
people and the environment," he says.
Advocates say the importance of
establishing effective nano-safety guidance is difficult to
overstate.
But that effort is also dauntingly
difficult.
"Get these rules wrong - and we're
not sure what they are yet - or ignore them, and we may cause
unnecessary harm to people and the environment," says Andrew
Maynard, chief science adviser for the Wilson Center.
"I don't think it will be the end of
the world as we know it. But it will be a lost opportunity to
get an exciting new technology right."
No One's in
Charge
The U.S. government has no nano czar, no single entity responsible
for setting priorities and doling out billions in research funds.
But on paper, the
National Nanotechnology Initiative
(NNI) comes closest
to fitting that role.
Launched by the Clinton administration's 2001 budget, the NNI was
tasked with coordinating federal investment in nanotechnology
research and development.
The official description of its mission
mandates it to "advance a world-class nanotechnology research and
development program, foster the transfer of new technologies into
products for commercial and public benefit, and support responsible
development of nanotechnology."
While that sounds somewhat czar-like, the reality is that who's in
charge of America's nanotech policy is murky.
"Final authority resides in the
[White House's] Office of Science and Technology Policy, the
Office of Management and Budget, and with the president," says
NNI Director Clayton Teague.
At the same time, Teague's position is
that there's no need for a central, government-wide coordinating
entity on nanotechnology.
"There is no nuclear 'czar,' no
independent authority over information technology, electronic
technology, or biotechnology for health and medicine," he says,
adding that nanotechnology activities "claim less than 1 percent
of the federal research and development budget" and therefore
"simply don't require the special focus you are suggesting."
NNI's biggest shortcoming, say even the
agency's supporters, is its failure to adequately fund basic
research on the safety hazards of nanomaterial.
"The NNI has never effectively
addressed environmental, health and safety issues surrounding
nanotech with a comprehensive, interagency plan," Matthew Nordan,
president of technology forecasting firm Lux Research, told the
Senate Committee on Commerce, Science and Transportation.
Although his statement was made almost
two years ago, committee investigators say there has been little or
no improvement since.
The Obama administration's 2011 budget illustrates the scant federal
resources devoted to nano-safety.
It proposes spending $1.8 billion on
nanotechnology overall, but just $117 million - or 6.6 percent - of
that was earmarked for the study of health-related issues
surrounding the engineered particles.
"It's not a small amount," says
Travis Earles, a nanotech adviser to the White House, defending
the allotment.
Without a single office leading the
charge, the task of guarding against potential nanotech risks falls
to the four agencies most involved in protecting the public, workers
and the environment:
-
the EPA
-
the Food and Drug Administration
-
the U.S. Department of
Agriculture
-
the Occupational Safety Health
Administration
Many of the safety experts in those
agencies told AOL News that the vital regulations for the use,
production, labeling, sale and ultimate disposal of nanomaterial are
not keeping pace with the rush of new products entering the
marketplace.
"Consumers want to know what they
buy, retailers have to know what they sell, and processors and
recyclers need to know what they handle," Christoph Meili, of
the Innovation Society Ltd., said in a report on international
nano regulations funded in part by the Swiss Federal Office for
the Environment.
Some of the scientists involved in
turning nanoparticles into new business opportunities, however,
argue that such protocols would be premature.
"I don't think we have the
scientific basis on which to establish regulations. And I think
that right now a lot of the materials that are being produced
are absolutely benign," says Stacey Harper, assistant professor
of nanotoxicology at Oregon State University.
"The Holy Grail," she says, "is figuring out what are those
[hazardous] features that we need to avoid in engineering these
newer materials."
'Do Nothing to
Prevent Innovation'
The FDA makes stringent demands for safety information on
nanomaterial used in medicine and medical devices, says Jesse
Goodman, its chief scientist and deputy commissioner for science
and public health.
But the agency takes no specific
measures to ensure the safety of the many costly cosmetics and
dietary supplements boasting the benefits of the nanoingredients
they claim to include, even though its own investigators say the
public submits a constant stream of questions and complaints.
"Nanotechnology products present
challenges similar to those the [FDA] faces for products of
other emerging technologies," says an agency press officer, and
"our existing regulations can pretty much handle these
advancements."

FDA headquarters in Rockville, Md.
Getty Images
The FDA
(whose Rockville, Md., headquarters are
shown here) has drawn fire from activists
for its approach to nanomaterials.
The
agency, says the Center for Food Safety's Jaydee Hanson, is "like ostriches with their
heads in the ground, not looking for a
problem so they do not see one."
That stated approach terrifies public
health advocates, as well as some of the agency's own risk
assessors.
"FDA is like ostriches with their
heads in the ground, not looking for a problem so they do not
see one. If they don't see one, they don't have to respond to a
problem," says Jaydee Hanson, senior policy analyst for the
Center for Food Safety.
The FDA does need better tools and
expertise to predict the behavior of nanomaterial, Goodman concedes.
But, he adds,
"to get information needed to assess
the safety of nano-products, we do that in a way that doesn't
cause a problem in terms of preventing innovation."
"Do nothing to prevent innovation" was former Vice President
Dick Cheney's marching orders to the Office of Management and
Budget during President George W. Bush's administration.
"For years OMB acted as industry's
protector," says Celeste Monforton, assistant research professor
at George Washington University's School of Public Health.
She is among the public health activists
who cringe to hear the phrase still being used by President Barack
Obama's regulators.
For all that, however, the FDA appears mostly AWOL in its handling
of nanomaterial in food. Food safety experts in the agency say it is
doing little more than paying bureaucratic lip service to developing
criteria for handling the anticipated avalanche of food, beverages
and related packaging that is heading to store shelves.
(The agency
declined repeated requests to interview any of its food scientists
or regulators.)
With the FDA largely punting, responsibility for ensuring the safety
of the nanomaterial in the marketplace falls to the Consumer Product
Safety Commission - which raises additional problems.
"If you take the nano-products that
we know are out there and divide them up among the safety
agencies, the CPSC is actually responsible for a majority of
those," says David Rejeski, science director for the Technology
Innovation Program at the Woodrow Wilson International Center
for Scholars.
In an analysis of CPSC's ability to
handle nanomaterial, Rejeski - who has worked in the White House
Office of Science and Technology Policy - and his team identified
many limitations.
The CPSC has no method of collecting
information on nano-products, and limited ability to inform the
public about health hazards.
"Even if they find a product,"
Rejeski says, "they don't have much ability to do any research
to determine whether it's dangerous."
Finding a Way Around
the Roadblocks
Since 2008, the EPA has been attempting to impose some controls on
carbon nanotubes, whose myriad industrial applications make them one
of the most heavily used engineered particles.
In June, it seemed to have made significant progress toward that
goal, issuing a final notice on a process called "Significant New
Use Rules," which would have required companies to notify the agency
at least 90 days prior to the manufacture, importing or processing
of carbon nanotubes.
The move was cheered by the public health community:
Studies have shown that multiwalled
nanotubes are among the nanomaterials posing potential risks to
humans, capable of damaging or destroying the immune system,
creating asbestos-like lung disease and causing cancer or
mutations in various cells.
The advocates heralded the measure as
the first clear sign that EPA was going to hold nano developers and
users accountable.
Almost immediately, the Washington law firm of Wilmer Hale, while
declining to say whom it represented, threw up procedural
roadblocks, notifying the EPA that it planned,
"to submit adverse and/or critical
comments on behalf of one or more clients."
That was enough to force the EPA to
withdraw the new rules.
The EPA resubmitted its proposal. But on December 1, in an unprecedented
move, the European Commission's Directorate-General for Enterprise
and Industry raised concerns on behalf of a British nanotube maker.
Action on the new rule was put off for another three months, with
the public comment period running through March.
Nevertheless, the EPA believes that by
year's end, its new nanotube requirements will be mandatory.

EPA Administrator Lisa Jackson in December
2009
EPA Administrator Lisa Jackson is pushing
for reforms that would make it mandatory for
companies to report the use of nanomaterials.
Industry players are pushing back.
Efforts such as those undertaken by
Wilmer Hale's client to stall or thwart new or enhanced safety
regulations are legal.
So is another practice used by many
corporations to deny EPA access to health studies and other
information crucial to assessing the risk of a new chemical or
product: declaring that the data is confidential business
information.
EPA Administrator Lisa Jackson has said she wants to put an
end to the corporate maneuvering, especially as it applies to the
new nanomaterial. While testifying before a Senate committee in
December attempting to add teeth to the Toxic Substances Control
Act, Jackson explained the obstacles EPA risk assessors confront in
trying to do their jobs.
Due to the legal and procedural hurdles in the law, over the past 30
years, the administrator said, EPA has only been able to require
testing on about 200 of the more than 80,000 chemicals produced and
used in the United States.
"EPA should have the clear authority
to establish safety standards that reflect the best available
science... without the delays and obstacles currently in place,
or excessive claims of confidential business information,"
Jackson told the lawmakers.
In February, the agency's assistant
inspector general, Wade Najjum, issued a report that said,
"EPA's procedures for handling
confidential business information requests are predisposed to
protect industry information rather than to provide public
access to health and safety studies."
The changes to the Toxic Substances
Control Act that Jackson is advocating would require mandatory
reporting of the use of nanomaterials.
EPA lawyers have told Senate
investigators that the overhaul is vital due to the industry
pressure spawned by the big business opportunities new nano-products
can generate. Meanwhile, some nanotechnology players are pushing
hard to get a resistant EPA to grandfather in nanomaterial already
on the market.
It's a significant point of dispute:
One of the reasons the EPA is
seeking the mandatory reporting requirement in the first place
is that the agency is convinced the current voluntary system of
submitting safety data doesn't work.
In the fall, EPA assistant administrator
Steve Owens told an international conference on regulating
nanomaterial that about 90 percent of the various nanoscale
materials already being used commercially, or thought to be used,
were never reported to the government.
"EPA has determined that regulating
existing nanoscale materials," explains press officer Dale
Kemery, "is needed to ensure protection of human health and the
environment."
A spokesman for the Senate Committee on
Environment and Public Works says two more hearings need to be held
on revising the Toxic Substances Control Act, but they have yet to
be scheduled.
Workers
Require Extra Protections
If there is a front-runner in the effort to institute meaningful
safety regulations for nanomaterial, it is the National Institute
for Occupational Safety and Health (NIOSH), the worker safety research arm
of the Centers for Disease Control and Prevention.
Physicians and scientists there have
been scrambling to identify the risks that the nanotechnology
industry's employees are encountering on the job.
"Workers and employers can't wait
for us to come up with all the answers before they unleash this
technology. It's unleashed already," says Paul Schulte, manager
of the NIOSH Nanotechnology Research Center.
Having published more than 170
peer-reviewed studies on the health effects from nano exposure, the
agency has established exposure limits for nano-titanium dioxide -
the heavily used material shown to damage and destroy DNA and
chromosomes in studies at UCLA.
The division has recommended that to
ensure safety, the exposure limit for workers handling nano-titanium
dioxide should be 15 times lower than that for the normal size of
the chemical, says Vincent Castranova, the agency's chief of
the Pathology and Physiology Research Branch.
The particles are believed to be used in more than 100 different
manufacturing sites across the country. That's a lot of workers.
NIOSH cannot pass laws, only make recommendations to the
Occupational Safety and Health Administration (OSHA). And because of all
the tortuous, bureaucratic steps that still must be completed, as
well as the anticipated blocking efforts from some industry
interests, it could be two years before any regulations are
instituted.
OSHA leaders refused to respond to
questions on what the agency will do in the meantime. NIOSH has also
almost completed recommendations for the handling of carbon
nanotubes.
And scientists at NIOSH's animal labs in
Morgantown, W.Va., are now testing the toxicity of almost two dozen
other nanoparticles, including,
-
the diesel additive cerium oxide
-
the metal hardening mixture of
tungsten carbide and cobalt
-
the anti-microbial agent
nanosilver
-
the sunblocker zinc oxide

Vial of nanotubes
Since 2008, the EPA has also been seeking to
impose some controls on carbon nanotubes.
That effort has been made more difficult by
corporate maneuvering.
Most significantly, NIOSH scientists
have identified health risks from nanomaterials not previously
documented by other researchers.
For example, says Castranova, when studying the potential impact of
nano-titanium dioxide exposure on workers' lungs, they also found
cardiovascular effects - damage to the heart muscle.
Separately, the NIOSH team discovered that beyond the
well-documented lung damage that comes from inhalation of carbon
nanotubes, those heavily used carbon structures were causing
inflammation of the brain in the test animals.
"Everything we say could apply to a
consumer. The big difference is that the consumer will likely
see much lower concentrations, for much shorter periods of
time," Castranova says, adding that the findings need to be
viewed with the proper perspective.
Nanomaterials, Castranova says, are not
anthrax, but they aren't Kool-Aid, either.
Other
Countries Exercise Greater Caution
Consumer and safety watchdogs say Canada, the U.K. and the rest of
the European Union are far ahead of the U.S. when it comes to nano
safety requirements.
Canada became the first country to demand stringent reporting
requirements of corporations and universities that import,
manufacture or use more than 2 pounds of nanomaterial a year. The
regulations - necessary for proper risk assessment, the Canadian
government said - were crafted and are enforced by Health Canada and
Environment Canada.
They require the reporting of the
nanomaterial's chemical composition and physical description,
toxicity and proposed use, along with other data.
French lawmakers have drafted legislation with similar stipulations.
And the European Parliament voted last year that its 27 member
states should consider nanomaterials as new substances, and not
cover them under existing laws that do not take into account the
risks associated with the technology.
It also demanded that consumer products
containing nanomaterials be clearly labeled as such, and that the
manufacturers of new cosmetic products containing nanomaterials
provide specific information to regulators six months before the
product is placed on the European market.
In the U.K., the battle cry of nano-regulators is "No data, no
market," especially with food products.
"Products will simply be denied
regulatory approval until further [safety] information is
available," the British House of Lords' science committee said
in December.
It concluded that there was too little
research into the toxicological impact of eating nanomaterials, and
too much secrecy on the part of the food industry.
"It's obvious that in some cases the
U.S. has been a bit lax, though you could make the case that in
some cases the EU requirements are a little bit too stringent,"
says Michael Holman, research director of Lux Research.
"There's no regulatory regime that
can give you 100 percent certainty that everything that comes to
market is going to be perfectly safe."
But to Patty Lovera, assistant
director of Food & Water Watch, that doesn't leave the U.S.
government off the hook.
The failure of the U.S. regulatory
system to keep up with nanotechnology, she states simply,
"puts consumers and the environment
at risk."
|












