|
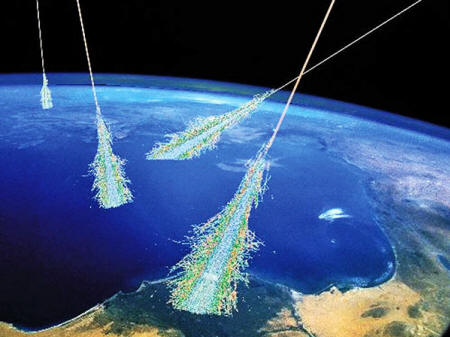
Cosmic rays are atom fragments that rain down on the Earth from outside of the solar system.
They blaze at the speed of light and have been blamed for electronics problems in satellites and other machinery. First discovered in 1912, many things about cosmic rays remain a mystery more than a century later. One prime example is exactly where they are coming from.
Most scientists suspect their origins
are related to supernovas (star explosions), but the challenge is
that cosmic ray origins appear uniform when you look across the
entire sky.
History
While cosmic rays were only discovered in the 1900s, scientists knew something mysterious was going on as early as the 1780s.
That's when French physicist Charles-Augustin de Coulomb - best known for having a unit of electrical charge named after him - observed an electrically charged sphere suddenly and mysteriously not being charged any more.
At the time, air was thought to be an insulator and not an electric conductor.
With more work, however, scientists discovered that air can conduct electricity if its molecules are charged or ionized. This would most commonly happen when the molecules interact with charged particles or X-rays.
But where these charged particles came from was a mystery; even attempts to block the charge with large amounts of lead were coming up empty.
On Aug. 7, 1912, physicist Victor Hess flew a high-altitude balloon to 17,400 feet (5,300 meters). He discovered three times more ionizing radiation there than on the ground, which meant the radiation had to be coming from outer space.
But tracing cosmic ray "origin stories" took more than a century.
In 2013, NASA's Fermi Gamma-ray Space Telescope released results from observing two supernova remnants in the Milky Way: IC 433 and W44.
Among the products of these star explosions are gamma-ray photons, which (unlike cosmic rays) are not affected by magnetic fields. The gamma rays studied had the same energy signature as subatomic particles called neutral pions.
Pions are produced when protons get stuck in a magnetic field inside the shockwave of the supernova and crash into each other.
In other words, the matching energy signatures showed that protons could move at fast enough speeds within supernovas to create cosmic rays.
Current science
We know today that galactic cosmic rays are atom fragments such as protons (positively charged particles), electrons (negatively charged particles) and atomic nuclei.
While we know now they can be created in supernovas, there may be other sources available for cosmic ray creation. It also isn't clear exactly how supernovas are able to make these cosmic rays so fast.
Cosmic rays constantly rain down on Earth, and while the high-energy "primary" rays collide with atoms in the Earth's upper atmosphere and rarely make it through to the ground, "secondary" particles are ejected from this collision and do reach us on the ground.
But by the time these cosmic rays get to Earth, it's impossible to trace where they came from. That's because their path has been changed as they travelled through multiple magnetic fields (the galaxy's, the solar system's and Earth's itself.)
According to NASA, cosmic rays therefore come equally from all directions of the sky.
So scientists are trying to trace back cosmic ray origins by looking at what the cosmic rays are made of. Scientists can figure this out by looking at the spectroscopic "signature" each nucleus gives off in radiation, and also by weighing the different isotopes (types) of elements that hit cosmic ray detectors.
The result, NASA adds, shows very common elements in the universe. Roughly 90 percent of cosmic ray nuclei are hydrogen (protons) and 9 percent are helium (alpha particles). Hydrogen and helium are the most abundant elements in the universe and the origin point for stars, galaxies and other large structures.
The remaining 1 percent are all elements, and it's from that 1 percent that scientists can best search for rare elements to make comparisons between different types of cosmic rays.
Scientists can also date the cosmic rays by looking at radioactive nuclei that decrease over time.
Measuring the "half life" of each nuclei gives an estimate of how long the cosmic ray has been out there in space.
Space radiation concerns
Earth's magnetic field and atmosphere shields the planet from 99.9 percent of the radiation from space. However, for people outside the protection of Earth's magnetic field, space radiation becomes a serious hazard.
An instrument aboard the Curiosity Mars rover during its 253-day cruise to Mars revealed that the radiation dose received by an astronaut on even the shortest Earth-Mars round trip would be about 0.66 sievert.
This amount is like receiving a whole-body CT scan every five or six days.
A dose of 1 sievert is associated with a 5.5 percent increase in the risk of fatal cancers. The normal daily radiation dose received by the average person living on Earth is 10 microsieverts (0.00001 sievert).
The moon has no atmosphere and a very weak magnetic field. Astronauts living there would have to provide their own protection, for example by burying their habitat underground.
The planet Mars has no global magnetic field. Particles from the sun have stripped away most of Mars' atmosphere, resulting in very poor protection against radiation at the surface.
The highest air pressure on Mars is equal to that at an altitude of 22 miles (35 kilometers) above the Earth's surface.
At low altitudes, Mars' atmosphere provides slightly better protection from space radiation.
|