|

November 9, 2011
from
TopSecretWriters Website
Why would a bright and promising cardiologist be fired from the
University hospital that she had practiced at since 2000?
Apparently, protecting her patients is grounds for dismissal. At
least, that is the case at Northwestern University in Illinois.
Despite being promoted to Valve Director in 2006, Dr. Nalini M.
Rajamannan (below image) was terminated in 2008 after reporting
the use of non-FDA approved, experimental medical devices being
implanted in patients without their knowledge.

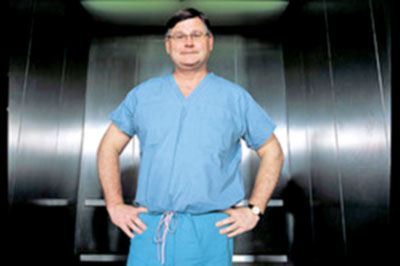
The doctor conducting these human
experiments, Dr. Patrick McCarthy (below image), was testing
his own inventions, an IMR annuloplasty device and a Myxo
annuloplasty device manufactured by
Edwards Lifescience.
These devices had not been approved by
the FDA and even now, many patients have no idea that they have
these experimental devices in their bodies.
One Patient’s
Experience
Dr. Rajamannan first discovered this deception in July 2007 when one
of her patients, Antonitsa Vlahoulis, required a second surgery to
replace the Myxo annuloplasty device less than a year after it was
implanted.
Ms. Vlahoulis questioned why the device she had was not the one
listed on her pre-op brochure.
Discussing her experience, Ms. Vlahoulis stated:
“When I had my consult with Dr.
McCarthy, he told me that I had severe Mitral valve prolapse. He
said with the severity of my valve, he would most likely have to
replace the valve with a pig valve or prosthetic.
He explained the difference between
the two. He also stated that his specialty was saving the valve
by repairing it with a mitral valve ring. He never mentioned
what type of ring, or that he was an inventor and he had
designed a ring for this purpose.”
She went on to say:
“He told me I would feel like a new
person immediately after my surgery.
I knew as soon as I woke up from the
surgery that I was in trouble. I did not feel like a new person.
My breathing felt a lot worse. I had a lot of complications only
to find out he had implanted a device he had just invented and
start implanting in patients one month before me.
I was never asked to sign an informed consent, nor was I advised
that I was part of an experimental trial.”
Ms. Vlahoulis said that by the time she
had the device removed, the experimental ring had caused stenosis.
She also needed her tricuspid valve
repaired and now has a permanent pacemaker. She says she continues
to have shortness of breath upon exertion as well as other heart
issues.
Her question remains unanswered:
“How can you place a non FDA device
in a patient without their consent and knowledge? What century
are we living in?”
Dr. Rajamannan
Seeks Answers
Dr. Rajamannan informed Chief of Cardiology Dr. Robert Bonow,
The Dean of Medical School Dean Jameson, the Institutional
Review Board and the NMFF general counsel that there was a human
clinical trial testing experimental devices without informed consent
in July 2007.
Her report fell on deaf ears, as the
University backed Dr. McCarthy (above image) and informed Dr.
Rajamannan that she would no longer be seeing McCarthy’s patients.
To make matters worse, Dr. Rajamannan discovered evidence that
negative outcomes of the experimental trials were not reported
in the process obtaining safety approval for Dr. McCarthy’s
inventions.
One patient, Ms. Maureen Obermeier, had a heart attack during
surgery, and the event wasn’t reported until five years later. Dr.
McCarthy had previously stated that no one had suffered a heart
attack.
Sadly, this isn’t the only case of
safety issues relating to the experimental devices.
Dr. Rajamannan states:
“I am trying to get the FDA and
Congress to open a Congressional investigation to discuss the
fact that the company and the surgeons did not provide the
outcomes to the FDA until recently.
The numbers were only a few hundred
prior to the Fall of 2009, now the numbers are over 4,000
adverse events on the MAUDE database and the total number of
deaths are 645. Patients are suffering from these human
experiments and no one has stepped up to help these patients to
date.”
Money Above
Human Life
Sadly, this isn’t the only case of human experimentation by Dr.
McCarthy (below image).

According to Dr. Rajamannan, Dr.
McCarthy has conducted at least four other human experiments,
two at Northwestern and two at Cleveland Clinic.
In the case of one of these experiments, Atricure Inc. was ordered
to pay $3.76 million in civil claims for its promotion of surgical
ablation devices that were not FDA approved.
What would motivate doctors, who make the oath to do no harm, to
play the part of a mad scientist and actually conduct secret
experiments on human beings?
Simply stated, the motivator is money.
Doctors receive royalties for medical devices that are tested and
approved. The added ego boost of being published in prestigious
medical journals may make doctors forget that their first loyalty
should be the well-being of their patients and not their bank
accounts.
So while Dr. McCarthy continues to practice medicine on unsuspecting
patients, apparently with an encouraging pat on the back from
Northwestern, Dr. Nalini Rajamannan continues to fight for her
patients and their right to know what was done to them.
When asked what she will do now that she has been terminated, Dr.
Rajamannan responded:
“My hope is that Northwestern
University Board of Trustees will review this situation and
restore everything that was terminated for my career and provide
a path for helping my patients who are suffering and restore the
integrity of the University.”
Top Secret Writers wish Dr. Rajamannan
the best of luck.
Note: Top Secret
Writers contacted Dr. McCarthy for his side of the story. He
declined to comment.
References
|



