|
Director 17 October 2011 from IMVA Website
Perhaps now that I am living in Sanctuary surrounded by two rivers, which envelop us on three sides, I will get back to my Waters of Life book and finally finish it.
Here we are reminded every day the
importance of water and what it’s like to drink it as it comes out
the top of a mountain. We do have a village water system for our
little valley of perhaps 70 simple homes but it is for collection
and no treatment is done.
Alkalinity also needs to be high for the
high pH water to have a strong medical affect.
Alkalinity is important because it
protects Alkalinity and pH are related to each other in ways that are obvious, and in other ways that are subtle.
The idea that alkalinity is separate from pH (which is by ‘coincidence’ called either acid or alkaline) is a myth though pH and alkalinity are two different measurable parameters of water.
Even though the pH can be very high we
find that un-mineralized water has little ability to neutralize acid
in the stomach to initiate the production of bicarbonate in the
bloodstream.
Alkalinity is a measure of the buffering
capacity of Alkalinity is the water’s capacity to resist changes in pH that would make the water more acidic.
This capacity is commonly known as
“buffering capacity.” For example, if you add the same weak acid
solution to two vials of water - both with a pH of 7, but one with
no buffering power (e.g. zero alkalinity) and the other with
buffering power (e.g. an alkalinity of 50 mg/l) - the pH of the zero
alkalinity water will immediately drop while the pH of the buffered
water will change very little or not at all.
pH simply expresses the degree of
hydrogen ion concentration. Alkalinity of natural water is determined by the soil and bedrock through which it passes.
The main sources for natural alkalinity are rocks which contain carbonate, bicarbonate, and hydroxide compounds. Borates, silicates, and phosphates also may contribute to alkalinity. Limestone is rich in carbonates, so waters flowing through limestone regions or bedrock containing carbonates generally have high alkalinity - hence good buffering capacity.
Conversely, areas rich in granites and
some conglomerates and sandstones may have low alkalinity and
therefore poor buffering capacity.
A pH less than 6.5 may contribute to the
corrosion of The pH level of drinking water is a measure of how acidic or basic it is - pH is related to the hydrogen ions in water and stands for “potential of hydrogen.”
Alkalinity is a measure of the capacity of water to neutralize acids. It measures the presence of carbon dioxide, bicarbonate, carbonate, and hydroxide ions that are naturally present in water. At normal drinking water pH levels, bicarbonate, and carbonate are the main contributors to alkalinity.
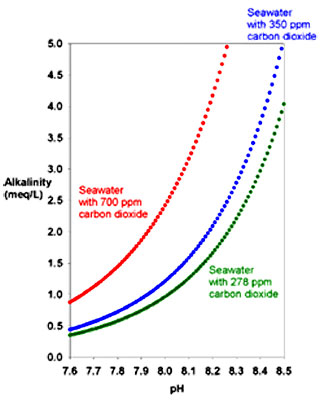
As we can see in the below graph the
higher the CO2 the more alkaline the water at a given pH.
In the chemistry of natural waters, there are several types of alkalinity that are encountered.
Each of these is a measure of how much acid (H+) is required to lower the pH to a specific level. The reason that aquarists measure alkalinity is that in normal seawater, most alkalinity consists of bicarbonate and carbonate. Consequently, alkalinity is an indication of whether or not adequate bicarbonate is present in the water.
Sodium bicarbonate is the main alkaline
buffer in our blood.
Alkaline supplied from outside the body, The main chemical species that contribute to alkalinity in seawater are bicarbonate and carbonate.
The table below (from “Chemical
Oceanography” by Frank Millero; 1996) shows the contribution to
alkalinity from the major contributors in seawater at pH 8.
Carbon dioxide has a specific solubility in water as carbonic acid (H2CO3).
At any given pH there is an exact mathematical relationship between H2CO3 and both bicarbonate and carbonate. For example, at a pH of about 9.3 in freshwater (about 8.4 in seawater) the carbonate concentration is 100 times that of the carbonic acid.
At higher pH this multiplier rises, and
there is consequently more bicarbonate and carbonate present.
alkalinity rises sharply as pH is
raised. This becomes
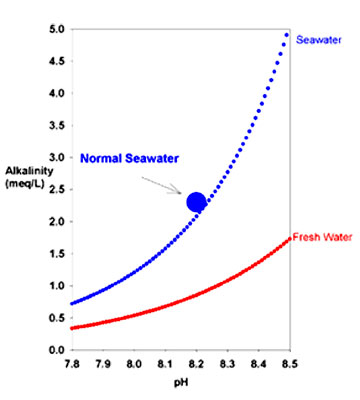
The theoretical relationship between
carbonate alkalinity and pH for seawater (blue) and Normal to high alkalinity implies adequate bicarbonate, while low alkalinity implies that it is in short supply.
Bicarbonated water is the healthiest water to drink and that is made clear in my Sodium Bicarbonate book. It is critical to see that alkalinity does not depend strictly on pH though. There is a relationship between the two but pH measures the degree of alkalinity but not its quantity. It is like the relationship between temperature and heat.
You can have a paper clip heated to
10,000 degrees but it will not heat a house nearly as well as
90-degree air blown from a home heater.
Alkalinity measures the concentrations
of bicarbonate, Alkaline ionizers do not always deliver water that is sufficiently acid-neutralizing to make a difference.
Alkaline ionizer promoters equate acid-neutralizing ability with high pH. From the discussion above we can see that it is the parameter of alkalinity that neutralizes acid, not pH levels alone. In other words you can have high pH and little alkalinity and you can have low pH and a lot of alkalinity (e.g. sparkling mineral water).
If there is only a small amount of
alkaline elements (from the first two columns of the Periodic Chart)
an ionizer will generate a meager quantity of acid neutralizing
alkalinity - but the pH will still show as a high alkaline value
(e.g. 8.5 to 10.5).
The presence of calcium carbonate or
other People living in low mineral areas (many city supplies and wells) think they are getting a good dose of alkalinity from their ionizers when they would be much better off with 1/2 tsp. of baking soda or a shot glass of Gerolsteiner Sparkling Water.
So it will be advised for many users of
water ionizers to add sodium bicarbonate to their water if they are
looking for stronger healing effects.
"Alkaline water" is not the same as
"water with alkalinity". For this The shortcoming of ionizers is simply that the input water chemistry determines its degree of benefit in terms of acid-neutralizing alkalinity (not pH!) and negative ORP (active hydrogen).
The quality of one’s “raw” water resource has to have a lot to do with our decision in terms of filters and ionizers chosen.
When the source water is low in minerals
(most public drinking waters are low in minerals especially
magnesium and bicarbonate) re-mineralization becomes critical.
Conclusions
Alkaline solutions, at about pH 8.5 has
been shown to overtly Sang Whang, one of the world’s great experts on reversing aging reminds us that,
NaCl + H2O + CO2 =
HCl + NaHCO3, or Wang says,
As we can see from the above chemical equations the byproduct of making hydrochloric acid is sodium bicarbonate (NaHCO3) or potassium bicarbonate (KHCO3).
In response to ingestion of sodium bicarbonate or high pH alkaline water the production of hydrochloric acid is actually increased because the stomach responds to lower the pH back down to normal acidic conditions. So as we take more alkalinity from drinking high pH and alkaline water, it forces our stomach to produce more acid (and a balancing amount of bicarbonate).
The bottom line is that a net gain of
alkalinity is achieved in the body and this is extremely helpful in
a body struggling to maintain equilibrium.
Notes
|