|
by John Rennie and Lucy Reading-Ikkanda
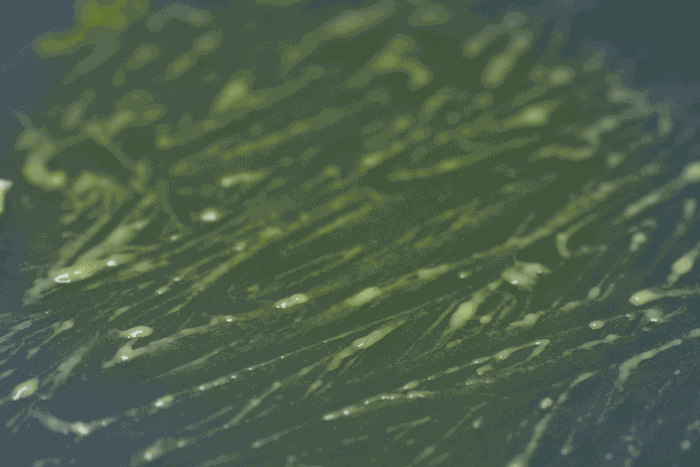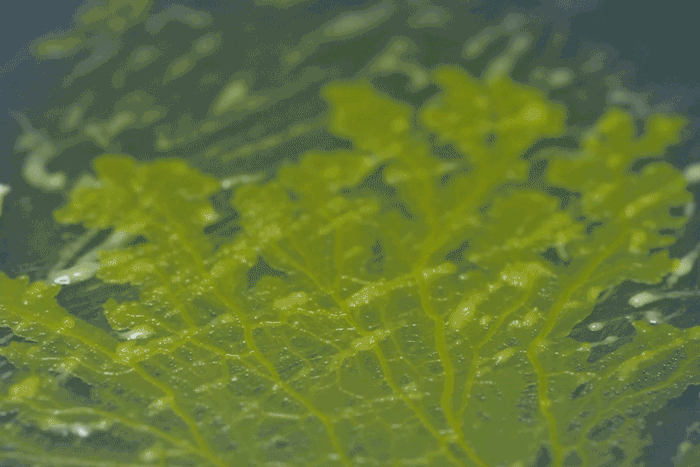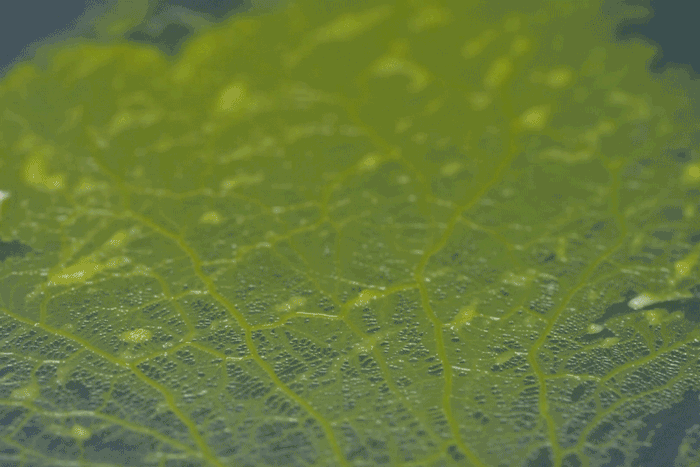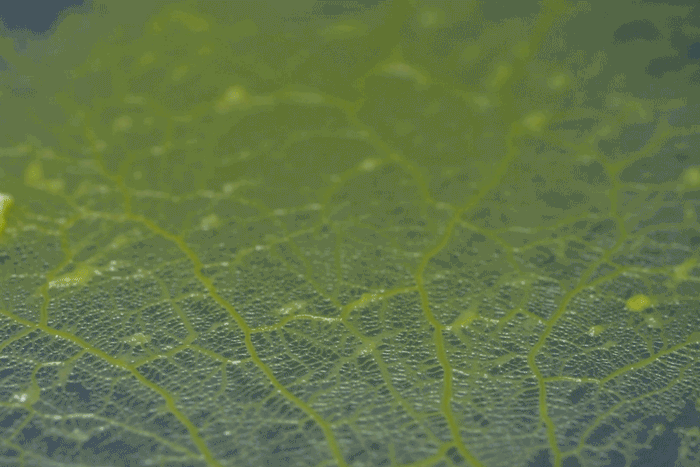
forms a network of cytoplasmic veins
as it spreads across a surface. Scott Chimileski and Roberto Kolter
(except where indicated) are more than crude patches of goo.
Detailed time-lapse microscopy reveals how they sense and explore their surroundings, communicate with their neighbors and adaptively reshape themselves...
Intelligence is not a quality to attribute lightly to microbes.
There is no reason to think that bacteria, slime molds and similar single-cell forms of life have awareness, understanding or other capacities implicit in real intellect.
But particularly when these cells commune in great numbers, their startling collective talents for solving problems and controlling their environment emerge.
Those behaviors may be genetically encoded into these cells by billions of years of evolution, but in that sense the cells are not so different from robots programmed to respond in sophisticated ways to their environment.
If we can speak of artificial intelligence for the latter, perhaps it’s not too outrageous to refer to the underappreciated cellular intelligence of the former.
Under the microscope, the incredible exercise of the cells’ collective intelligence reveals itself with spectacular beauty.
Since 1983, Roberto Kolter, a professor of microbiology and immunobiology at Harvard Medical School and co-director of the Microbial Sciences Initiative, has led a laboratory that has studied these phenomena.
In more recent years, it has also developed techniques for visualizing them.
In the photographic essay book Life at the Edge of Sight - A Photographic Exploration of the Microbial World released in September, Kolter and his co-author, Scott Chimileski, a research fellow and imaging specialist in his lab, offer an appreciation of microorganisms that is both scientific and artistic, and that gives a glimpse of the cellular wonders that are literally underfoot. Imagery from the lab is also on display in the exhibition World in a Drop at the Harvard Museum of Natural History.
That display will close in early January but will be followed by a broader exhibition, Microbial Life, scheduled to open in February.
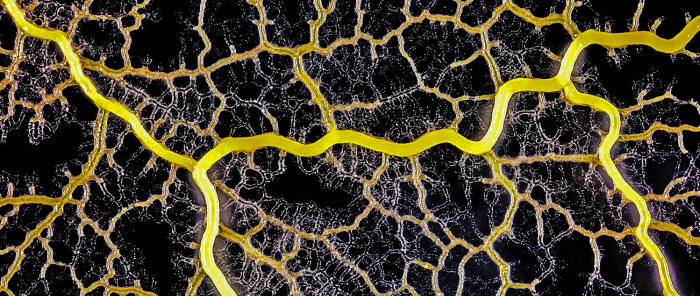
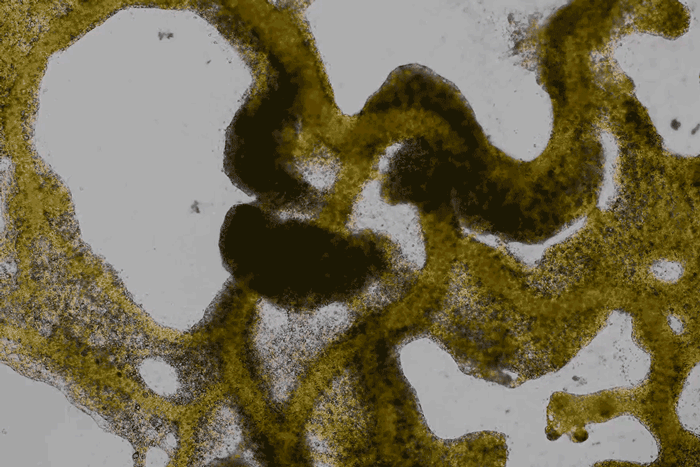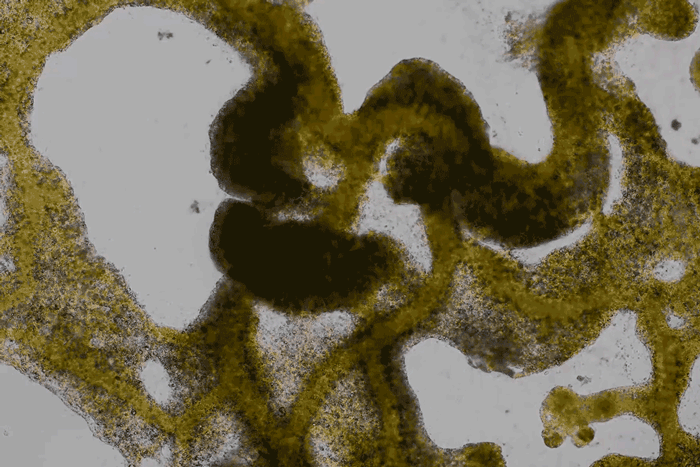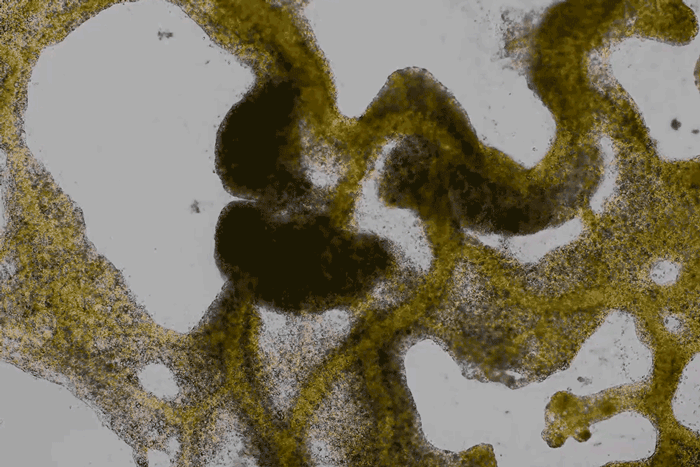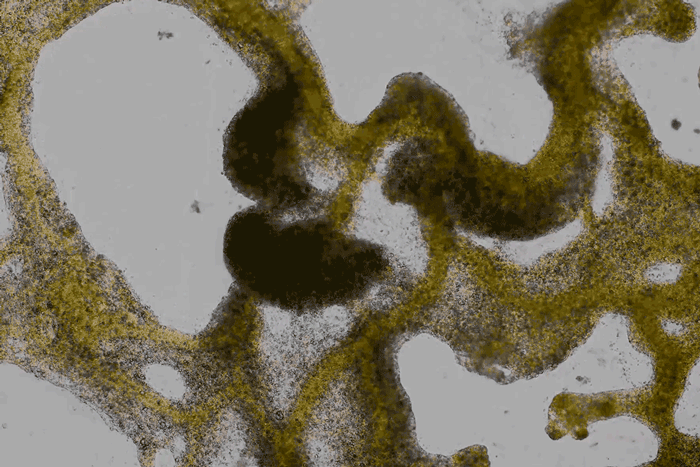
High magnification of the slime mold Physarum polycephalum shows the cytoplasm pumping furiously through its huge single cell. This cytoplasmic streaming allows the slime mold
to push
forward toward nutrients and potentially carpet a surface. The slime mold Physarum polycephalum sometimes barely qualifies as a microorganism at all:
Yet despite its size, Physarum is a huge single cell, with tens of thousands of nuclei floating in an uninterrupted mass of cytoplasm. In this form, Physarum is a superbly efficient hunter.
When sensors on its cell membrane detect good sources of nutrients, contractile networks of proteins (closely related to the ones found in human muscle) start pumping streams of cytoplasm in that direction, advancing the slime mold toward what it needs.
But Physarum is not just reflexively surging toward food. As it moves in one direction, signals transmitted throughout the cell discourage it from pushing counterproductively along less promising routes.
Moreover, slime molds have evolved a system for essentially mapping their terrain and memorizing where not to go:
After Physarum explores an area and finds it lacking in nutrients, it leaves behind a chemical trail as a kind of externalized memory that tells the slime mold not to go back there.
When bacteria were first observed through a microscope, suspended in liquid on slides, in their simplicity they seemed like the archetypes of primitive, solitary cells.
The truth, however, is that in the wild, most bacteria are highly gregarious.
Some bacteria do swim through their environment as lonely individuals but most bacterial cells - and most species of bacteria - prefer to live in compact societies called biofilms anchored to surfaces.
(The individual swimmers often represent offshoots of biofilms, seeking to colonize new locations.)
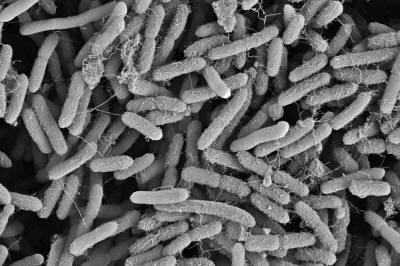
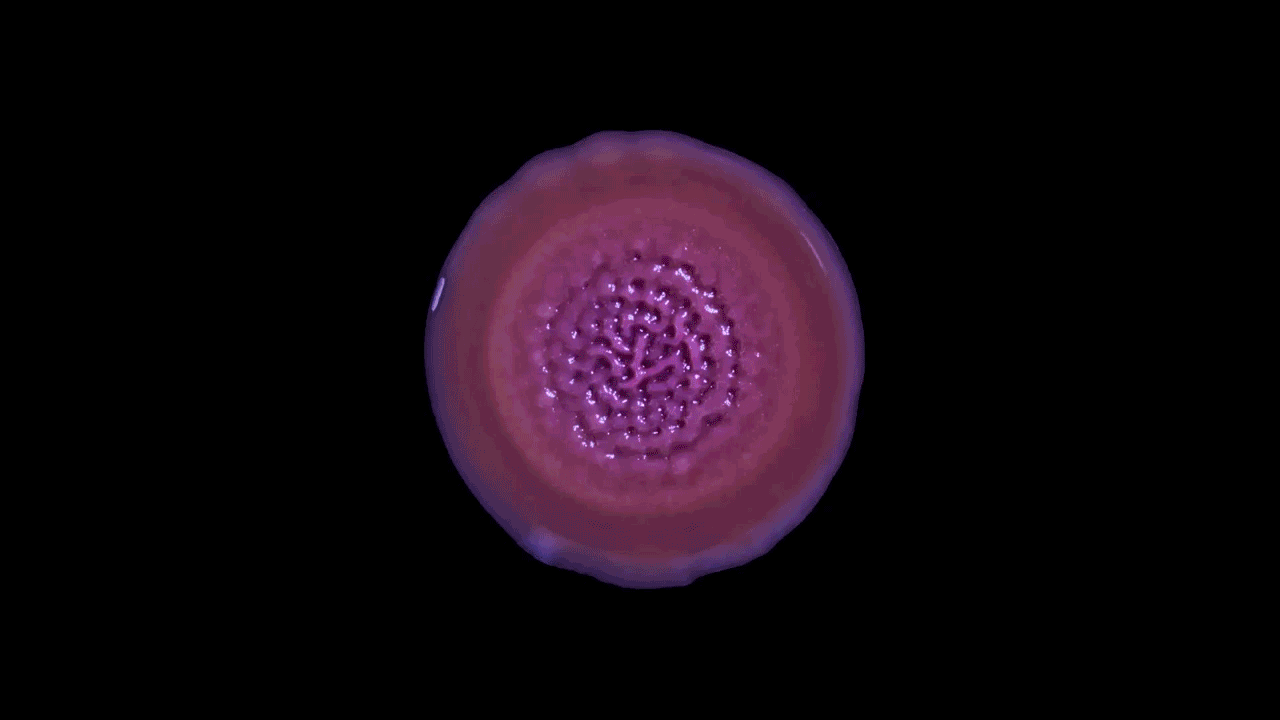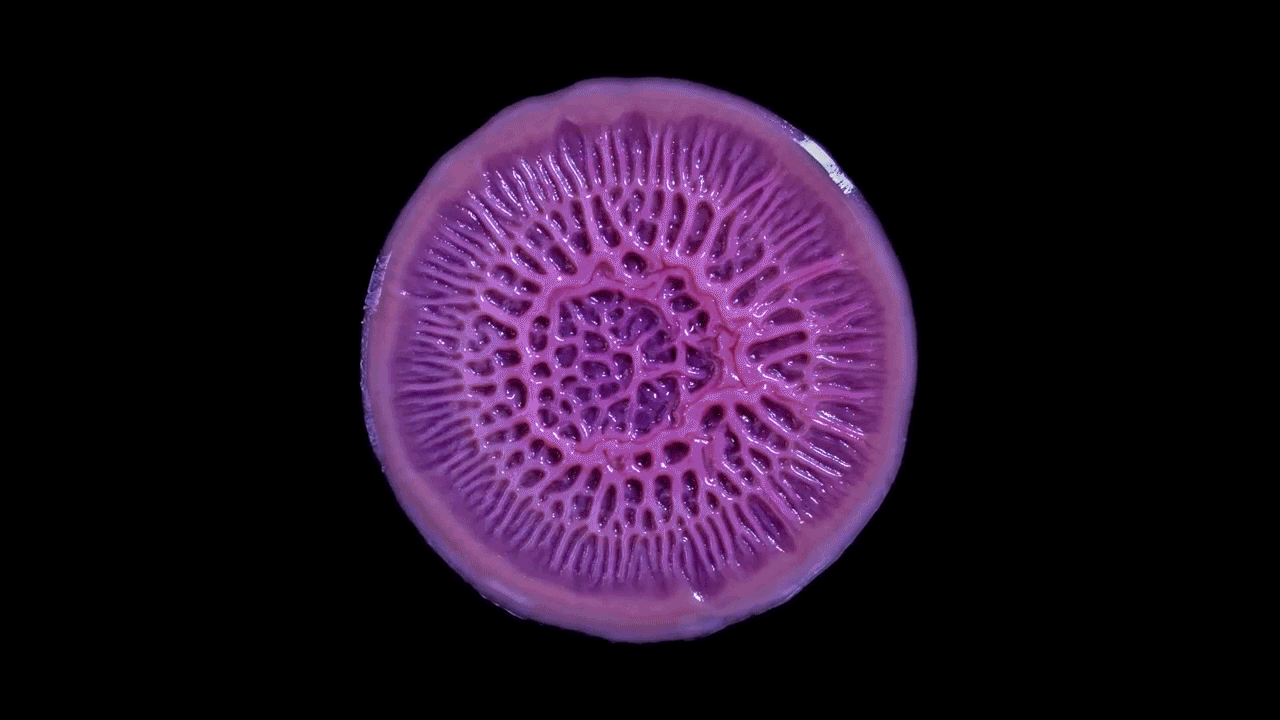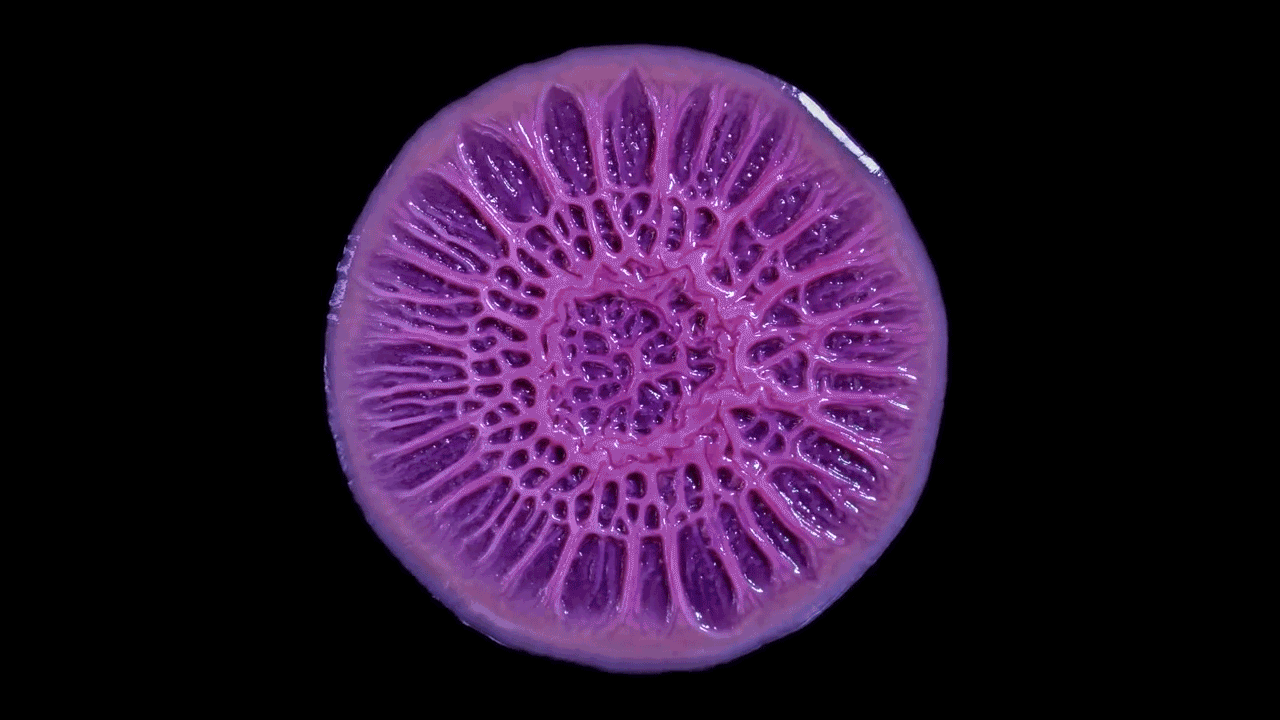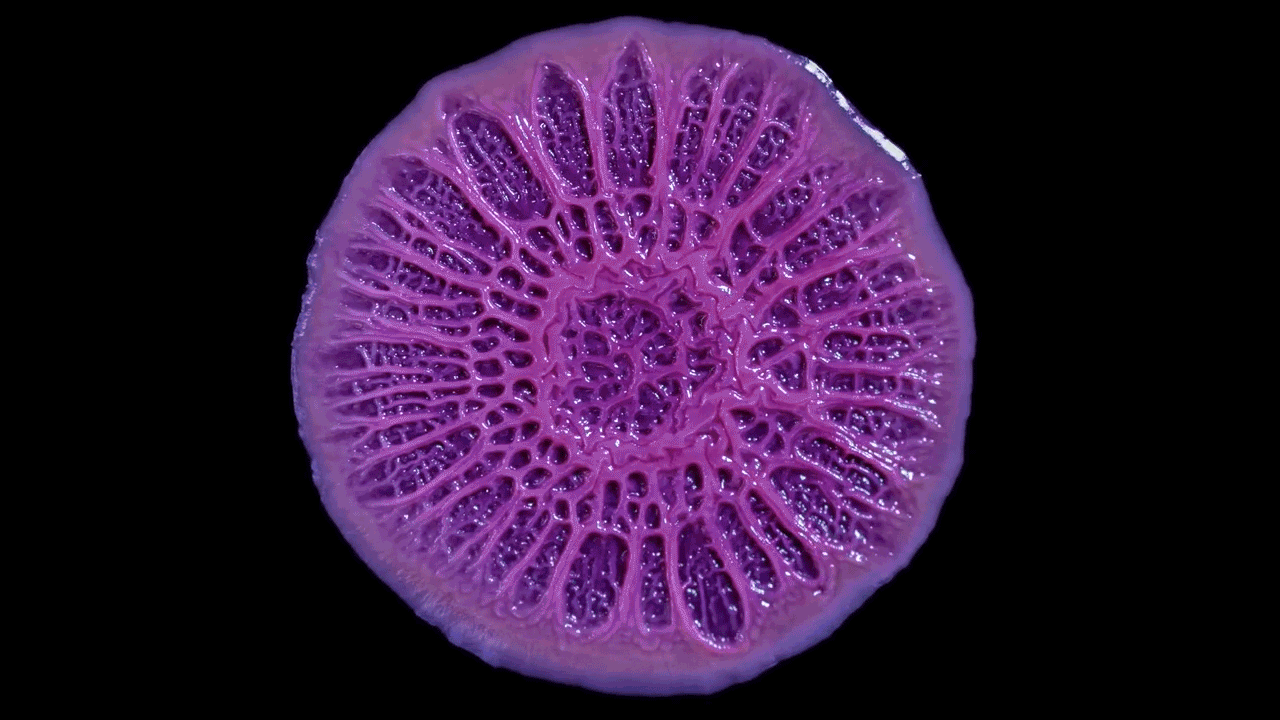
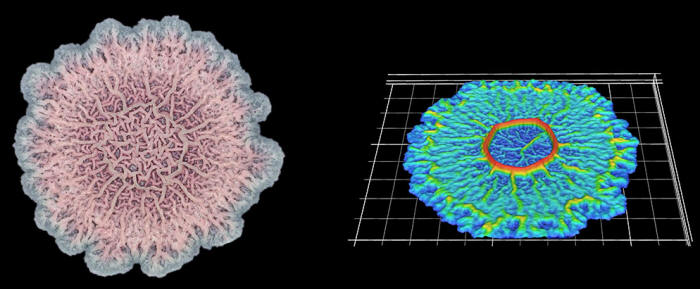
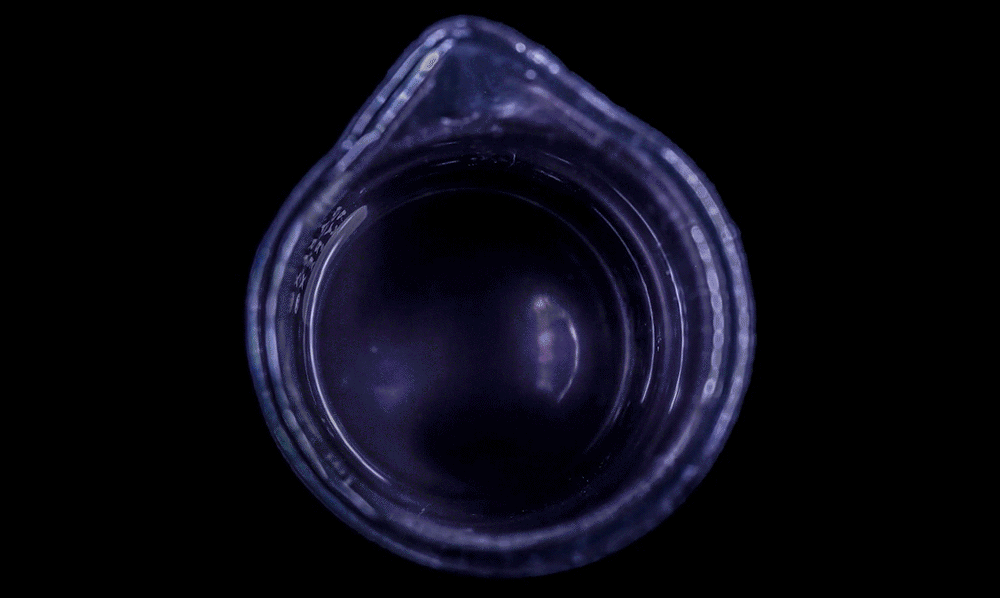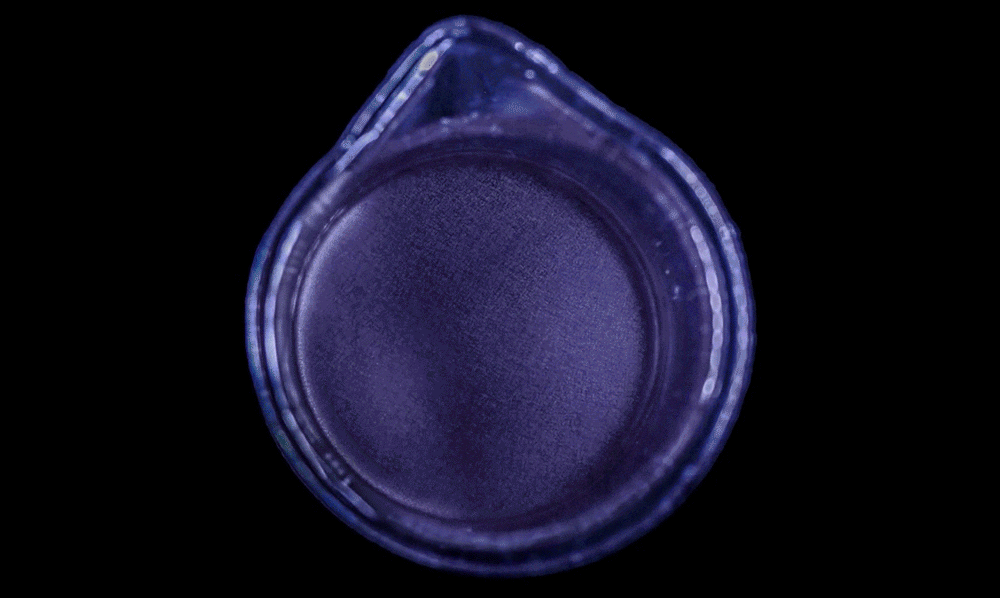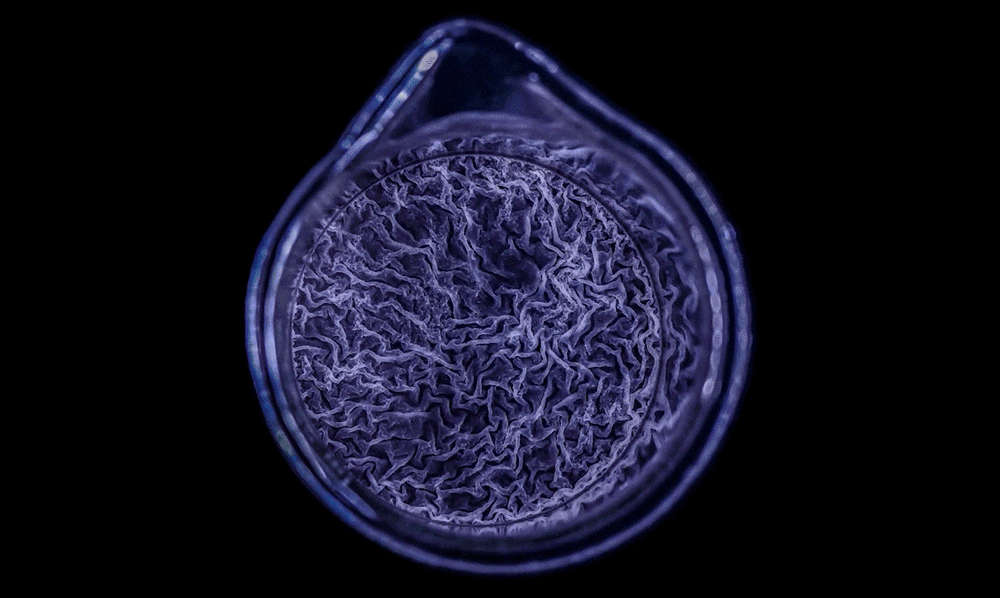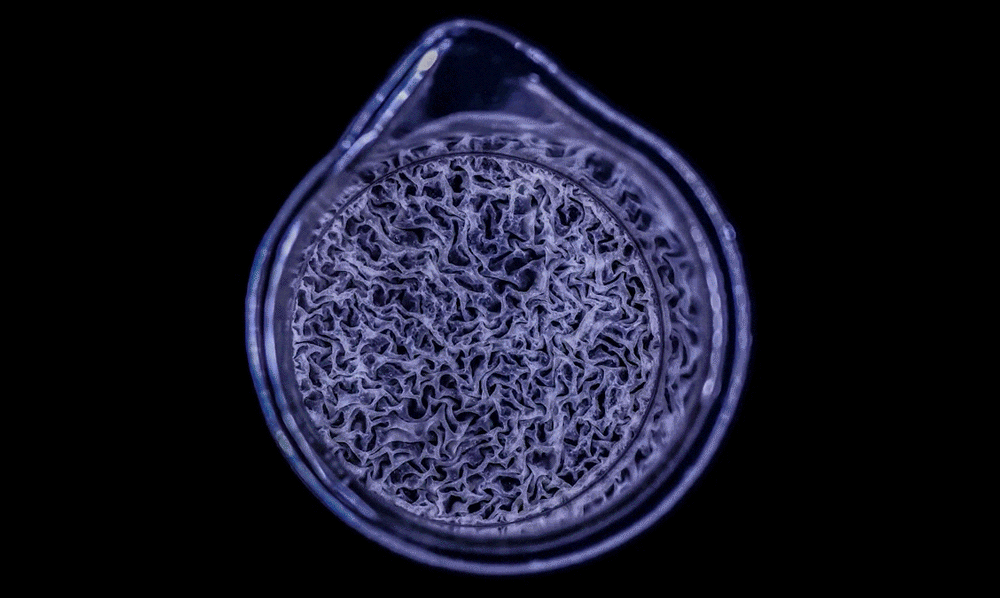
In a high-magnification scanning electron micrograph of a Pseudomonas aeruginosa biofilm, the individual rod-shaped bacteria are interlinked by hairlike structures called pili. Bacillus bacteria secrete an extracellular matrix that encases the cells and helps them form
a more structured
community.
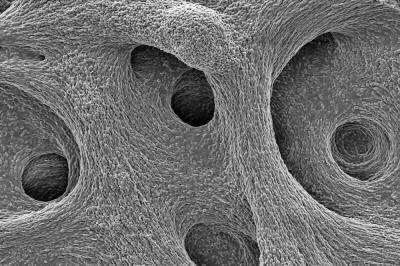
Moreover, biofilms are not just dense accumulations of bacterial cells.
The biofilm is stained with Congo red dye, which bonds to the extracellular matrix proteins that the bacteria secrete as a scaffolding for their community.
The deeply wrinkled surface of the biofilm maximizes the area through which the bacteria can absorb oxygen; it also probably helps them collect nutrients and release waste products efficiently.
As this Pseudomonas biofilm expands, it develops a more complex internal structure. Bacteria in different parts of its mass may also develop more specialized functions.
Within the biofilm, the bacteria divide the labor of maintaining the colony and differentiate into forms specialized for their function.
In this biofilm of the common soil bacterium Bacillus subtilis, for example, some cells secrete extracellular matrix and anchor in place, while some stay motile.
Cells at the edges of the biofilm may divide for growth, while others in the middle release spores for surviving tough conditions and colonizing new locations.
The wrinkled structure of this Bacillus subtilis biofilm helps to ensure that all the bacteria in it have access to oxygen (left). A digital scanned model of the biofilm helps illustrate how the bacterial community can vary its structure in three dimensions (right).
One might wonder why natural selection would have favored this collective behavior instead of more rampant individualism among the cells.
Part of the answer might be what evolutionary theorists call inclusive fitness:
But it may also be that every role within the biofilm has its advantages:
Cells on the inside depend on others for their vital rations but they may survive longer.
The surfaces that biofilms grow across are not always solid. These B. subtilis are forming a pellicle - a kind of floating biofilm at the interface between water and air.
The genetic pathways involved in forming a pellicle are essentially the same as those used in growing across stones, though they may respond to the changes in their habitat by altering the precise mix of proteins in the extracellular matrix as needed.
Bacteria can grow across nonsolid surfaces, too, as this B. subtilis culture shows by forming a pellicle, or floating biofilm, across the air-liquid interface in a beaker.
Expansive growth is not the only way in which microbial communities can move.
Below, B. subtilis is engaging in a behavior called dendritic swarming, in which cells rapidly push outward in branching columns that can efficiently pave a surface.
Biofilms swarm when they detect that they are in environments rich in nutrients:
At least two important changes in the differentiation of the cells in a biofilm take place to enable swarming.
First, motile cells on the periphery of the film develop extra flagellae, which enables them to swim more energetically. Second, some edge cells also begin to secrete surfactant, a slippery material that helps the motile cells slide more rapidly over the surface.
When biofilms grow in flat laboratory dishes, the dendritic columns of swarming biofilms remain neatly distinct:
That seems to be in part because the surfactant piles up around the biofilm branches as a barrier. Similarly, some bacteria can swarm in more terraced structures under laboratory conditions.
What the implications of that option are for bacteria in nature is still a mystery.
These bacteria are engaging in the behavior called dendritic swarming, which allows a microbial community to expand rapidly into desirable, resource-rich environments.
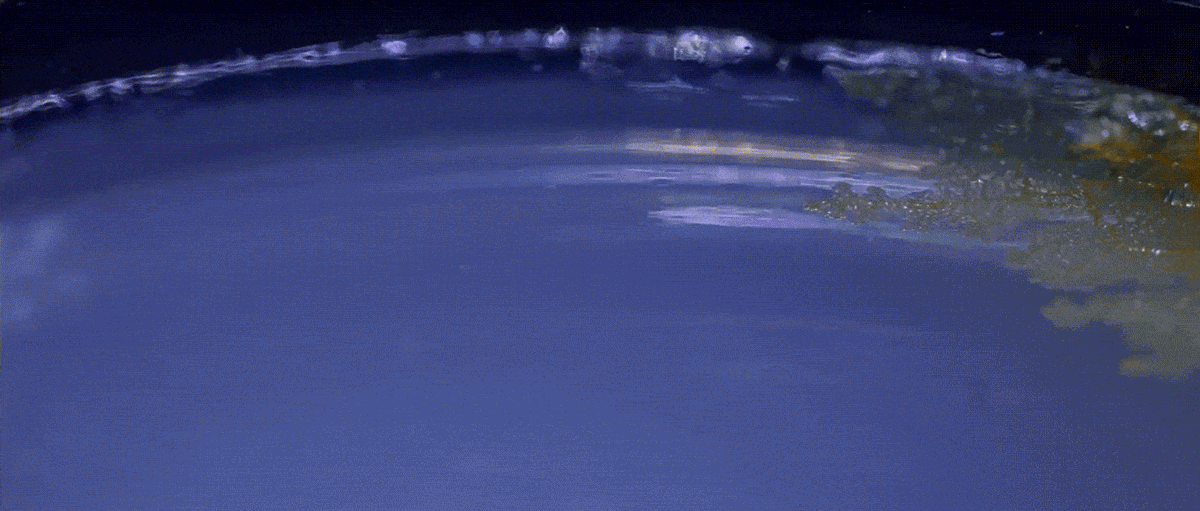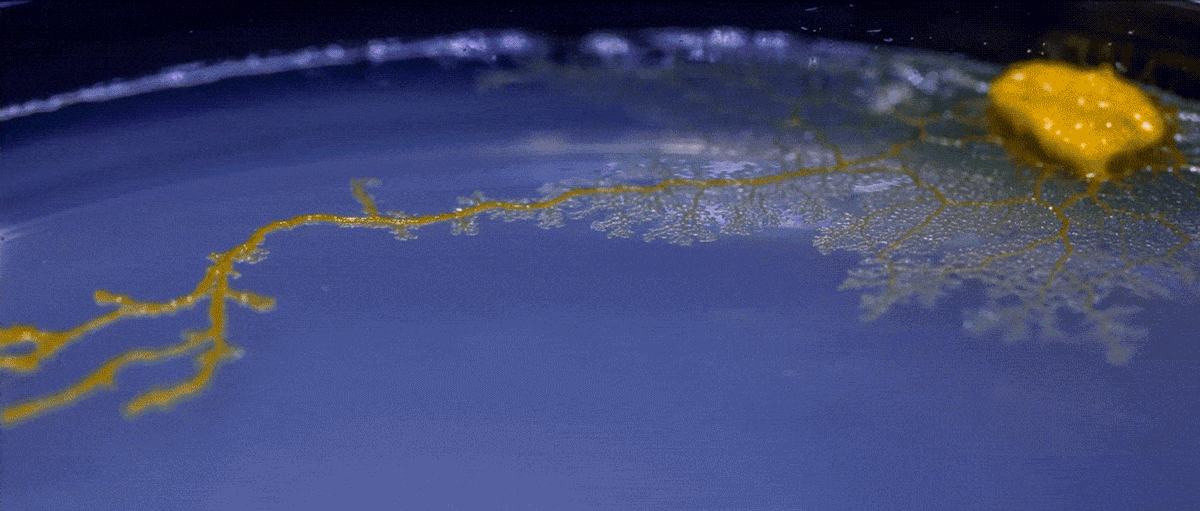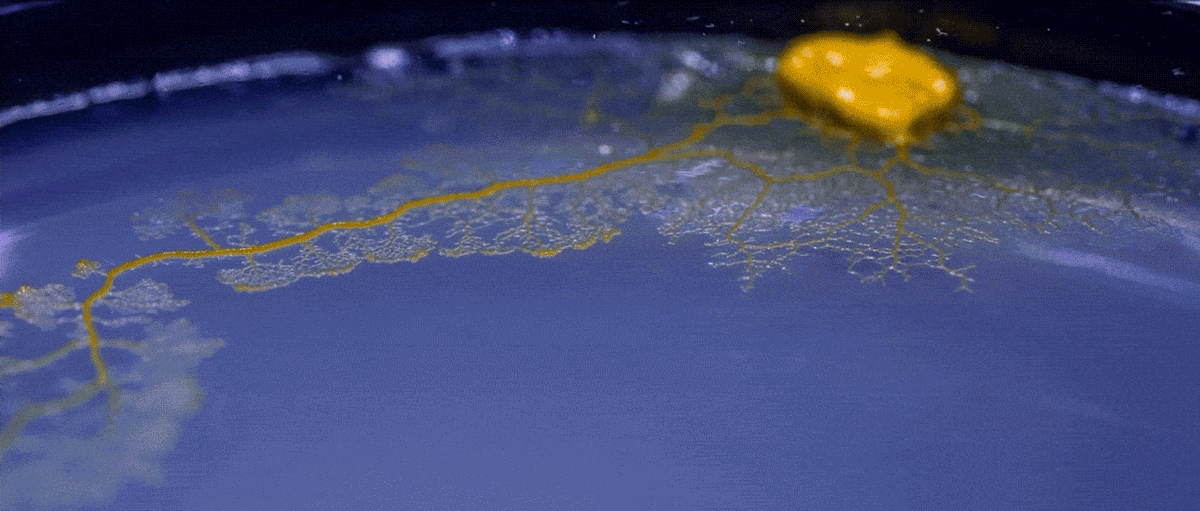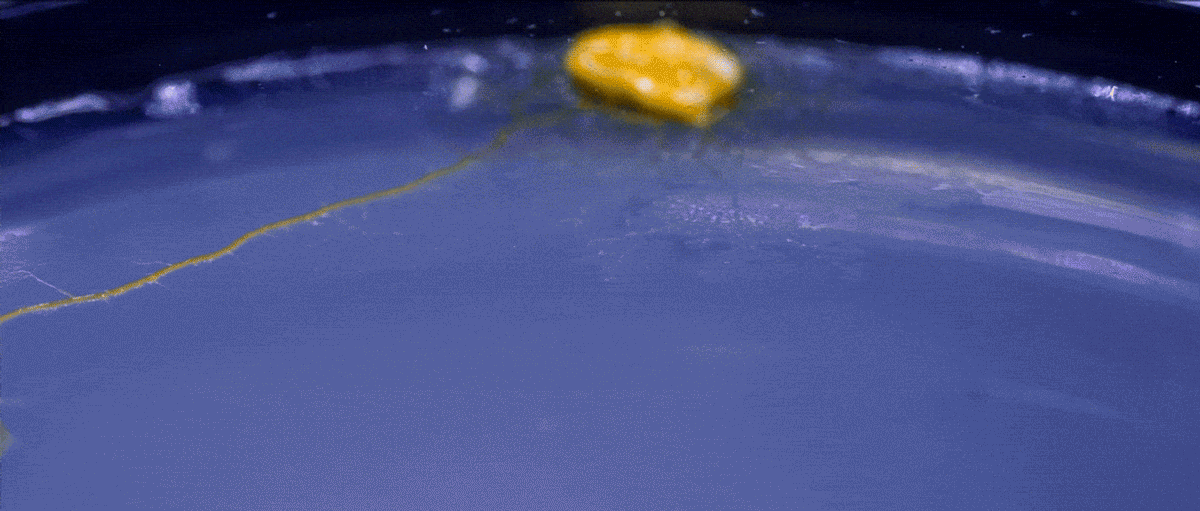
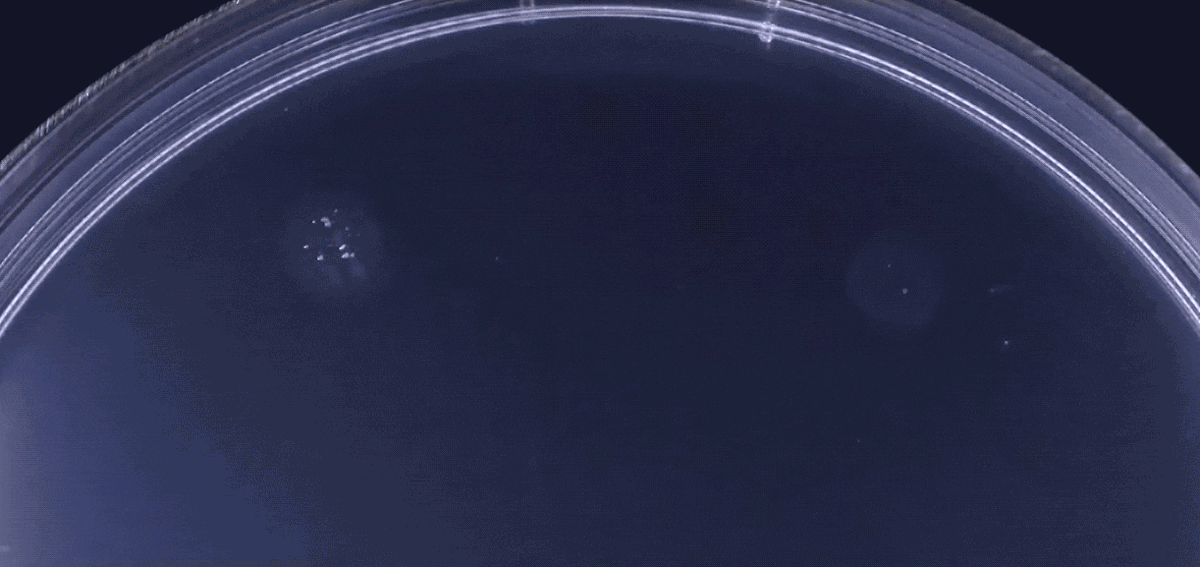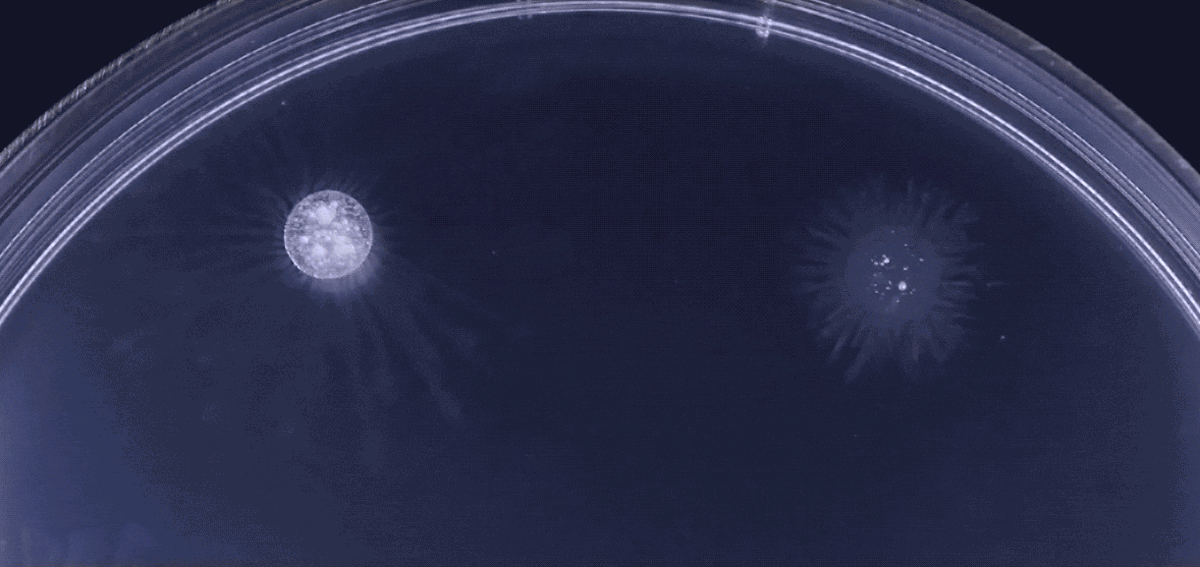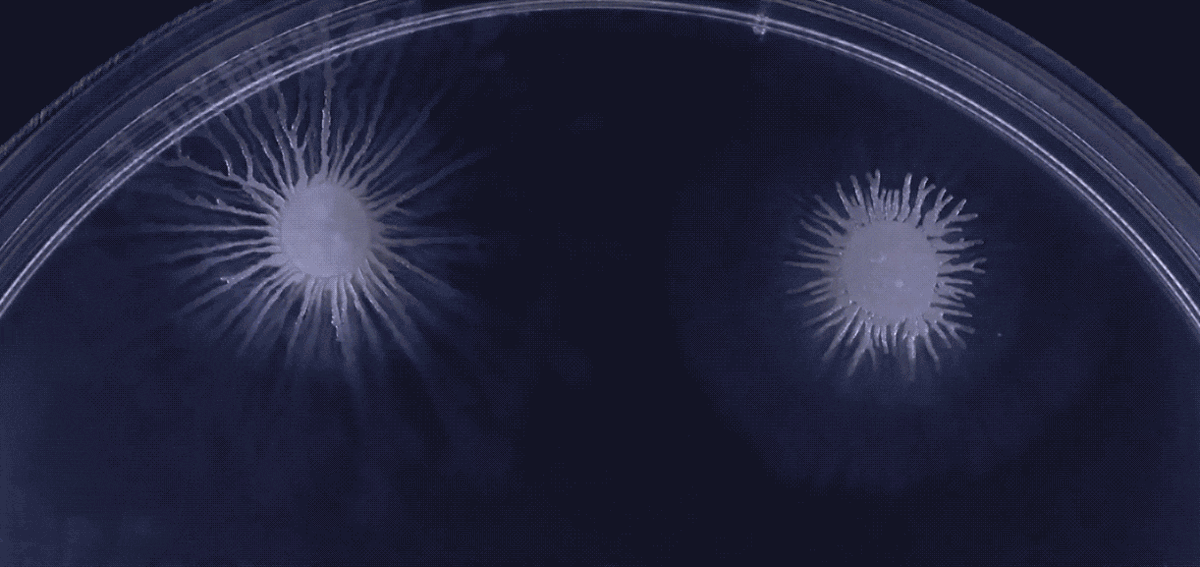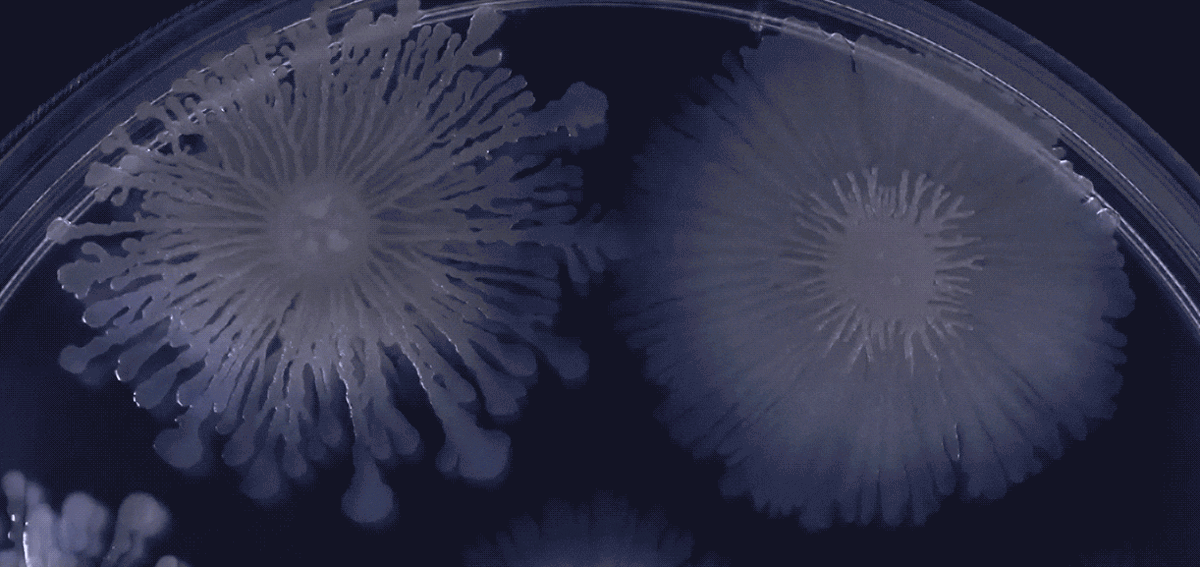
Another type of behavior demonstrated by biofilms growing under laboratory conditions is spiral migration, demonstrated in the time-lapse video below of Bacillus mycoides.
These bacterial cells grow in long chains or filaments that curl either clockwise or counterclockwise.
The specific advantages of this spiraling movement are still under investigation, according to Chimileski, but they must be considerable because B. mycoides excels at taking over available environments.
When scientists isolate microbes from soil and grow them on agar dishes, particularly at room temperature,
What’s curious is that the direction of the spiraling migration - clockwise or counterclockwise - seems to be a hereditary trait:
It is yet another example of how bacteria, obeying instructions in their individual DNA, can manifest problem-solving behaviors that are surprisingly complex and adaptive at the collective level of biofilms.
These geometric and presumably functional patterns that biofilms produce in culture are intriguingly beautiful. Yet Chimileski notes that there is much left to discover when it comes to translating behaviors seen in the lab to natural microbial communities.
Chimileski points out that,
He continued,
Under laboratory conditions, researchers can study which genes are involved in complex multicellular behaviors and they can measure the benefits to the fitness of the bacterial species.
But in natural environments, biofilms don’t usually get to form exactly the same patterns as in the lab because of limited nutrients or competition with other species.
Spiral migration is a behavior favored by the highly successful soil bacterium Bacillus mycoides. Communities of these cells expand by forming long filaments of cells that coil either clockwise or counterclockwise - an orientation that is strain-specific and genetically determined.
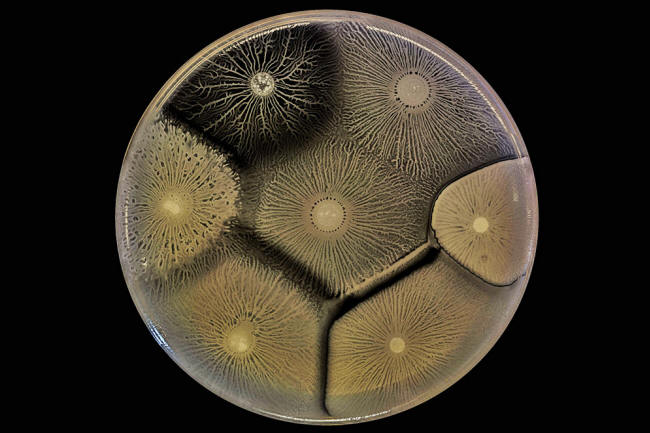
Biofilm behaviors testify to the capacity and openness of bacterial to form collectives - but that openness has limits, as shown in this culture with several cohabiting biofilms.
Here, adjacent biofilms that consist of the same bacteria or closely related strains comfortably merge. But the adjacent biofilms made up of more divergent bacteria keep themselves distinct and may even try to eliminate or control each other.
Biofilms are so intolerant of other strains and species because they invest considerably in the production of surfactant, extracellular matrix and other molecules that bacteriologists classify as public goods - ones that the bacteria secrete for other members of their community.
The bacteria guard these jealously because unrelated freeloading cells could benefit strongly by using them first.
Biofilms rebuff such freeloaders in different ways.
For example, the B. subtilis colonies in this image adopt a strategy of "kin discrimination," in which they secrete antibiotic compounds that are toxic to other species but not to their own.
Proteus mirabilis bacteria defend their interests in a different way based on "self-recognition":
Several different strains of B. subtilis grow side by side in this dish. Because the biofilms discriminate against dissimilar strains of bacteria, they may merge compatibly with close relatives but form boundaries against others.
The colors appearing in the biofilm culture of Streptomyces coelicolor in the video below reflect natural pigments that the bacteria produce.
The value of the pigments for the biofilms is not entirely clear, but it is probably not tied to their color.
Rather, these pigment molecules are often bioactive in various ways.
For that reason, he said, there is,
That view is echoed in a note from Gleb Pishchany, another research fellow in Kolter’s laboratory who studies how diverse types of bacteria cohabit.
The pigments may help cohabiting assortments of bacteria rein in one another’s less neighborly instincts, and thereby maintain a more cooperative and fruitful communal existence.
In this powdery colony of Streptomyces coelicolor, the pigmentation comes from actinorhodin, a molecule with antibacterial effects. Biofilms may use bioactive pigments as signals for controlling the behaviors of other microorganisms in their shared environment.
These striking photographs of microbe communities were captured by DSLR cameras.
Chimileski collects his still images with macro lenses while working at the bench, while the videos are made in an incubator dedicated to time-lapse microscopy.
He sets the camera to snap a picture every 10 minutes, although he increases the frequency to every minute or two for behaviors happening more quickly, such as the movements of slime molds.
As a result, the movements of the microbes in these videos are typically accelerated between 5,000 and 50,000 times their actual speeds.
Chimileski does not use false color to beautify the images:
Chimileski typically grows bacterial colonies at 30°C, a temperature at which he can collect images of slower growing species for several weeks.
Although the heat and humidity suited to biofilm growth are less than ideal for cameras, he said the equipment is rated for more extreme conditions.
The few cameras that have malfunctioned did so for a mechanical reason:
|