|

by Dr. Marian
Laderoute
June 14, 2023
from
HERV-K102 Website

For PDF click above
image...
If you think Big Pharma
had good reasons
to censor Ivermectin
during
COVID-19
how about now when we know
it is likely effective against
all chronic diseases associated with aging?
Dr. Paul Marik recently quoted a prospective clinical trial where
the participants were given 4000 international units of
Vitamin D,
omega 3's and told to exercise and the risk of cancer dropped 50%.

In another post, Dr. Marik says,
it is highly unlikely cancer is
genetically determined...
I wanted to talk about
both in this post.
In July 1994, I published a new theory of cancer in Molecular
Carcinogenesis which implied while tumors were genetically
determined, the malignant nature of tumors (i.e., the thing
we call cancer) was not. 1
This notion that malignancy was a phenotype and not a genotype was
heretical at the time and so the paper was ignored (only the editors
of Molecular Carcinogenesis and myself were excited).
It was an exciting idea
because it meant one can control the malignant potential of tumors
pharmacologically. No more need for slash and burn, which I have
always regarded as barbaric.
However, subsequently it became widely accepted that the malignant
phenotype of cancers called 'epithelial mesenchymal transition' (EMT)
was real. 2
So I was vindicated,
although it seems no one noticed except for myself.
The reason I had proposed cancer as a phenotype was that I had just
finished the characterization of the 67 kD alpha-fetoprotein
(AFP) receptor for my Ph.D. thesis. 3-6
The AFP receptor (AFPr)
4 was expressed on macrophages 3,6 and highly
over-expressed on the common cancers the adenocarcinomas (breast,
prostate, lung, colon, etc) 3,5 implying a dual role in
immuno-suppression of the host and in tumor malignant potential. In
fact I wrote the theory to explain how immuno-suppression of the
host relates to tumor malignant potential.
AFP was the very first immunosuppressive factor described (1974) and
the first tumor marker discovered (1963).
We were first to show it
prevented cell lysis (apoptosis) of macrophages in 1994
6 where the data had also been presented in the Ph.D. thesis
by 1991.
It is now well established that AFP contributes to the malignant
phenotype through the PI3K/Atk /mToR pathway, the same one conferred
by oncogenic HER-2/nu (ERB2).
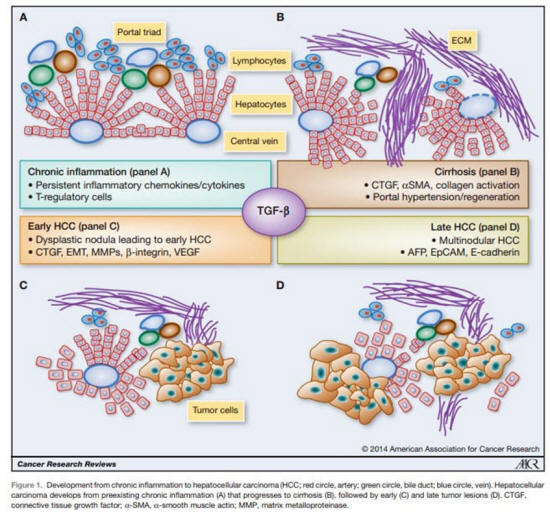
Figure 1.
From Giannelli G et al, Cancer Research 2014.
7
AFP is recognized as a malignancy progression factor
in
a very common cancer in the world,
hepatocellular carcinoma (HCC)
which is often associated with a viral origin.
Thus, the malignant potential of tumors known as cancer was amenable
to pharmacological intervention and by entities that inactivate AFP.
AFP exists in active and inactive states.
Things that bind to and
inactivate AFP (zinc, DHEA, flavonoids etc) are entities that may
help promote innate immunity (of macrophages) and which also
diminish the malignant potential of tumors.
At the time I called this malignant phenotype of cancers
"anti-cellular senescence". 1
This was because the
tumor was refractory to new signaling because AFP binding to the
AFPr on tumors blocked incoming signals. So it seemed the tumor did
not age. {Note anti-aging at the cellular level is associated with
aging at the whole host level.}
While the malignant phenotype is now recognized as 'epithelial-mesenchymal
transition' (EMT), fortunately Dr. Robert Weinberg has included the
phenotype of blocking senescence in EMT 8 ie., a
phenotype involving anti-cellular senescence (whew!).
Then in 2015, I wrote the new immuno-senescence paradigm of
macrophages published in Discovery Medicine (Figure 2) which
attempts to explain the cause of chronic illness associated with
aging including diseases such as cancer and cardiovascular diseases.
9
Noteworthy, the immuno-senescence of macrophages involves
immuno-suppression AND paradoxically the uncontrolled release of
pro-inflammatory factors such as IL-6, TNF-alpha and IL-1beta. It is
the inflammation that contributes to the start of chronic diseases.
This would be exacerbated
by ongoing infections due to the immuno-suppression.
AFP by binding the 67 kD AFPr, induces immunosuppression and
prevents the down-modulation of the inflammatory factors. It is
believed the inflammation is through activation of NFKB1 (see
later).
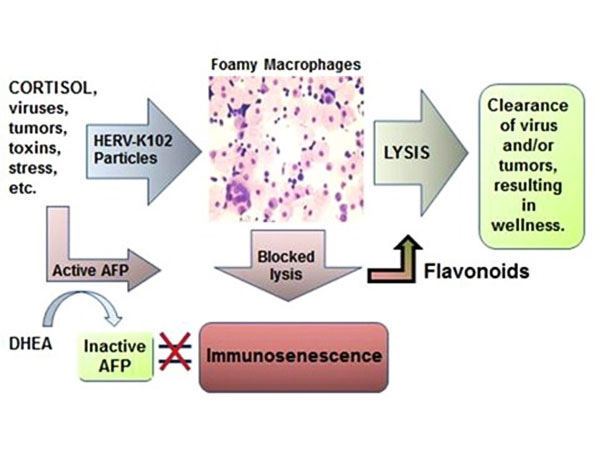
Figure 2.
The New Immunosenescence Paradigm of Macrophages
was defined as the failed (lytic) release of
protector HERV-K102 particles from foamy macrophages.
HERV-K102 is a non-pathogenic, protector foamy retrovirus
unique
to humans which renders macrophages foamy
(the
particles accumulate in vacuoles in
the cytoplasm giving the cells a foamy appearance).
When
the DHEA/cortisol ratio is low, there is a higher risk
of immunosenescence upon exposures to virus
due to inadequate levels of DHEA to bind and inactivate AFP.
We reported in 1994 that
AFP blocks apoptosis of macrophages [6].
Subsequently I validated this paradigm specifically for explaining
the initiation and progression of cardiovascular disease. 10
(i.e., it is not elevated cholesterol but elevated stress does
increase cholesterol so increases in cholesterol are a sign of
stress).
P53 along with TGF-beta, represses AFP expression, and p53 is a
tumor suppressor commonly deleted or at least dysfunctional in
tumors/cancers. 11
ZBTB20 is a zinc finger credited with post-natal down-regulation of
AFP. However, when functional p53 is absent, it upregulates AFP.
12
Did you know that ZBTB20 is required for the induction of NFKB1
13 such as when SARS-CoV-2 infects foamy macrophages in vivo
by antibody dependent enhancement of infection (ADE) 14
which contributes to cytokine storm?
AFP is significantly
upregulated by SARS-CoV-2 infection at the protein and mRNA level in
cell lines. 15
Referring back to Figure 2, AFP antagonists reverse and prevent
immuno-senescence.
Immuno-senescence causes
age-associated chronic diseases. 9,10
This means AFP
antagonists like zinc, flavonoids, DHEA/7ketoDHEA, Vitamin D over 60
ng/ml, and now also Ivermectin [16] are likely AFP antagonists which
are therefore, predicted to reduce the risks of age-associated
chronic illness including cancer and cardiovascular disease, etc..
There are now in addition to many reports on how Ivermectin behaves
as a potent antiviral, emerging evidence for its reversion of the
malignant phenotype particularly by inducing apoptosis 17
and blocking metastasis. 18
So if you think the war on Ivermectin is over, in fact, it is
just getting STARTED...!
REFERENCES
-
Laderoute MP. A
new perspective on the nature of the cancer problem:
anti-cellular senescence. Mol Carcinog. 1994
Jul;10(3):125-33. doi: 10.1002/mc.2940100303.
-
Zhang Y, Weinberg
RA. Epithelial-to-mesenchymal transition in cancer:
complexity and opportunities. Front Med. 2018
Aug;12(4):361-373. doi: 10.1007/s11684-018-0656-6.
-
Laderoute MP. The
Characterization of a Novel, Widespread, PNA-Reactive Tumor
Associated Antigen: the Alpha-fetoprotein Receptor/Binding
Protein. Ph.D. Thesis. The University of Alberta. Canada
1991, pp 256.
https://era.library.ualberta.ca/items/6f548eb6-49a2-456c-b472-41f68976077f.
-
Moro R, Tamaoki
T, Wegmann TG, Longnecker BM, Laderoute M. Monoclonal
antibodies directed against a widespread oncofetal antigen:
the alpha-fetoprotein receptor. Tumor Biology 1993 14
(2):116-130. Https: //doi.org/10.1159/000217864.
-
Laderoute M,
Willans D, Wegmann T, Longenecker M. The identification,
isolation and characterization of a 67 kilodalton, PNA-reactive
autoantigen commonly expressed in human adenocarcinomas.
Anticancer Res. 1994 May-Jun;14(3B):1233-45.
-
Laderoute MP,
Pilarski LM. The inhibition of apoptosis by
alpha-fetoprotein (AFP) and the role of AFP receptors in
anti-cellular senescence. Anticancer Res. 1994
Nov-Dec;14(6B):2429-38.
-
Giannelli G,
Villa E, Lahn M. Transforming growth factor-β as a
therapeutic target in hepatocellular carcinoma. Cancer Res.
2014 Apr 1;74(7):1890-4. doi: 10.1158/0008-5472.CAN-14-0243.
-
Weinberg RA.
Twisted epithelial-mesenchymal transition blocks senescence.
Nat Cell Biol. 2008 Sep;10(9):1021-3. doi:
10.1038/ncb0908-1021.
-
Laderoute MP. A
new paradigm about HERV-K102 particle production and blocked
release to explain cortisol mediated immunosenescence and
age-associated risk of chronic disease. Discov Med. 2015
Dec;20(112):379-91.
-
Laderoute M. The
paradigm of immunosenescence in
atherosclerosis-cardiovascular disease (ASCVD). Discov Med.
2020 Jan-Feb;29(156):41-51.
-
Wilkinson DS,
Ogden SK, Stratton SA, Piechan JL, Nguyen TT, Smulian GA,
Barton MC. A direct intersection between p53 and
transforming growth factor beta pathways targets chromatin
modification and transcription repression of the
alpha-fetoprotein gene. Mol Cell Biol. 2005
Feb;25(3):1200-12. doi: 10.1128/MCB.25.3.1200-1212.2005.
-
To JC, Chiu AP,
Tschida BR, Lo LH, Chiu CH, Li XX, Kuka TP, Linden MA, Amin
K, Chan WC, Bell JB, Moriarity BS, Largaespada DA, Keng VW.
ZBTB20 regulates WNT/CTNNB1 signalling pathway by
suppressing PPARG during hepatocellular carcinoma
tumourigenesis. JHEP Rep. 2020 Dec 19;3(2):100223. doi:
10.1016/j.jhepr.2020.100223.
-
Liu X, Zhang P,
Bao Y, Han Y, Wang Y, Zhang Q, Zhan Z, Meng J, Li Y, Li N,
Zhang WJ, Cao X. Zinc finger protein ZBTB20 promotes
Toll-like receptor-triggered innate immune responses by
repressing IκBα gene transcription. Proc Natl Acad Sci U S
A. 2013 Jul 2;110(27):11097-102. doi:
10.1073/pnas.1301257110.
-
Ren X, Wen W, Fan
X, et al. COVID-19 immune features revealed by a large-scale
single-cell transcriptome atlas. Cell. 2021 Apr
1;184(7):1895-1913.e19. doi: 10.1016/j.cell.2021.01.053.
-
Appelberg S,
Gupta S, Svensson Akusjńrvi S, et al. Dysregulation in Akt/mTOR/HIF-1
signaling identified by proteo-transcriptomics of SARS-CoV-2
infected cells. Emerg Microbes Infect. 2020
Dec;9(1):1748-1760. doi: 10.1080/22221751.2020.1799723.
-
Laderoute M.
Ivermectin may prevent and reverse immunosenescence by
antagonizing alpha-fetoprotein and downmodulating PI3K/Akt/mTOR
hyperactivity. Open Heart. April 29, 2021.
https://openheart.bmj.com/content/8/1/e001655.responses#Ivermectin-may-prevent-and-reverse-immunosenescence-by-antagonizing-alpha-fetoprotein-and-downmodulating-pi3k-akt-mtor-hyperactivity.
-
Tang M, Hu X,
Wang Y, Yao X, Zhang W, Yu C, Cheng F, Li J, Fang Q.
Ivermectin, a potential anticancer drug derived from an
antiparasitic drug. Pharmacol Res. 2021 Jan;163:105207. doi:
10.1016/j.phrs.2020.105207.
-
Jiang L, Sun YJ,
Song XH, Sun YY, Yang WY, Li J, Wu YJ. Ivermectin inhibits
tumor metastasis by regulating the Wnt/β-catenin/integrin
β1/FAK signaling pathway. Am J Cancer Res. 2022 Oct
15;12(10):4502-4519.
|