|
Robert A. Freitas Jr., Xenology:
An Introduction to the Scientific Study of Extraterrestrial Life,
Intelligence, and Civilization, First Edition, Xenology Research
Institute, Sacramento, CA, 1979;
http://www.xenology.info/Xeno.htm
(c) 1979 Robert A. Freitas Jr.
All Rights Reserved. |
Chapter 7.
The Origin of Life
"Who knows for certain? Who shall declare it?
Whence was it born, whence came creation?
The gods are later than this world’s formation;
Who then can know the origins of the world?
None knows whence creation arose;
And whether he has or has not made it;
He who surveys it from the lofty skies,
Only he knows -- or perhaps he knows not."
-- Rig Veda, ca. 1000
B.C.
"If a dirty undergarment is squeezed into the mouth of
a vessel containing wheat within a few days (say 21) a ferment drained from
the garments and transformed by the smell of the grain, encrusts the wheat itself
with its skin and turns it into mice. And what is more remarkable, the mice
from corn and undergarments are neither weanlings nor sucklings nor premature,
but they jump out fully formed."
-- Jan Baptista van Helmont
(1577-1644)2481
"It is often said that all the conditions for the first
production of a living organism are now present, which could have been present.
But if (and oh! what a big if!) we could conceive in some warm little pond,
with all sorts of ammonia and phosphoric salts, light, heat, electricity, etc.,
present, that a proteine compound was chemically formed ready to undergo still
more complex changes, at the present day such matter would be instantly devoured
or absorbed, which would not have been the case before living creatures were
formed."
-- Charles Robert Darwin (1809-1882)1017
"Ultimately, such a scientist is saying that man’s
mind was created by a batch of dancing chemicals. He is saying that Shakespeare
and St. Francis of Assisi were manufactured by something like Alka-Seltzer fizzing
in a glass."
-- in
The Sign, a
Catholic monthly (1956)114
"The molecules that could not copy themselves did not.
Those that could, did. The number of copying molecules greatly increased..."
-- Carl Sagan, in The
Cosmic Connection (1973)15
Scientists today will still admit that they really don’t
know how life began on our planet. Laboratory work is tricky, and nobody was
present to witness events at first hand on the primitive Earth. Researchers
in abiogenesis can only invent some reasonable story about how life arose, and
then maximize its plausibility by theoretical and experimental investigations.20
There are two central themes that run as undercurrents throughout
the whole of xenobiology. First, what is the probability that life of our kind
will evolve on other worlds? By illuminating the abiogenic processes of this
planet in ancient times, scientists hope to get a handle on the exact combination
of conditions and events necessary for the origin of carbon-based Earthlike
life anywhere in the Galaxy.
The second central theme of xenobiology, to which we shall
return in later chapters, is the likelihood that life, once having emerged in
a planetary environment, will constitute a form of biota more or less similar
to that found on Earth. The laws of biochemistry demand that molecules combine
only in certain specific ways, and usually only in a very few most probable
ways. In other words, what are the physical and biochemical limits of the possible?
7.1
Historical Views on the Origin of Life
Speculations on the source of life have been abundant throughout
recorded history. The Rig Veda mentions that biology began from the
primary elements, and the Atharva Veda suggests that the oceans were
the cradle of life. The Bible, with its contradictory accounts of the Creation
in Genesis (did man arrive before or after the beasts?), is strictly adhered
to by many fundamentalists.
Philip Henry Gosse, an eminent 19th century zoologist
and Christian, found it a simple task to reconcile the growing mass of paleontological
evidence with the Scriptures. God, he declared, created the Earth entirely in
accordance with scientific findings. The Lord fabricated geological strata,
embedded fossils and the like for the sole purpose of fooling geologists. The
apparently extreme age of Earth is only an illusion.
Peculiar ideas abound. Hylozoism, for instance, is the belief
that matter and life are one and inseparable. From this viewpoint, life either
has no origin and has always existed, or else the question may be deferred to
the origin of all matter.
The theory of pyrozoa, to cite another example, was advanced
by William Preyer in the last century. Preyer believed that life has existed
at all times, even when our planet was still in the molten state. These first
fiery living things, the pyrozoa, slowly modified and adapted themselves as
the environment cooled and changed, eventually assuming the form in which life
presents itself to us today.2218
Most theories on the origin of life have fallen into one of
four distinct categories:
1. Life has no origin -- both life and matter have existed
forever;
2. Life is the consequence of a supernatural event, intractable
and in explicable by the methods of science;
3. Life originated via ordinary chemical evolution in a
deterministic fashion -- under similar circumstances, the same general evolutionary
patterns would repeat themselves on any world; and
4. Life originated elsewhere by means unknown, and was
subsequently transported to Earth (panspermia).
The first two are self-explanatory, and the third closely
approximates the leading modern theories. The last deserves a word of explanation.
The Greek philosopher Anaxagoras of Clazomenae (ca. 500-428
B.C.) was possibly the first to suggest that the seeds of life permeated the
universe. With the downfall of spontaneous generation millennia later, panspermia
enjoyed a brief revival. The theory was sponsored by many 19th-century notables,
including Richter, Kelvin, Helmholtz, Arrhenius, and the great Italian chemist
Avogadro.
The doctrine of lithopanspermia held that meteorites were
the means by which life wandered from planet to planet throughout the cosmos.
Lord Kelvin, a central proponent of this view, considered it probable that countless
life-bearing "stones" existed in space, perhaps as the result of collisions
between inhabited worlds. Hermann von Helmholtz, a German philosopher and a
pioneer in physics, believed that the interior of a meteorite would be a safe
retreat for interplanetary microbes during the long incandescent journey through
thick planetary atmospheres. The presence of hydrocarbons in the carbonaceous
chondrites was cited as evidence of the biological activities of the tiny organisms
from space.
Modern analyses suggest that microscopic lifeforms embedded
in interstellar comets are possible, but unlikely. The accumulated radiation
dose from cosmic rays and natural internal radioactivity is "embarrassingly
high" over the large transit times involved between worlds.22
Furthermore, it is now known that meteorites are of roughly the same age as
the rest of the solar system, and that the organic molecules found in chondrites
are reproducible by strictly chemical means.208,2030,2219
The famous Swedish physical chemist and Nobelist Svante Arrhenius
was the loudest advocate for the theory of radiopanspermia.2304,2305,2306
He suggested that minute spores might be carried upward through planetary atmospheres
by convection, where electrical forces could provide sufficient energy to expel
them from the body. The pressure of sunlight would then be enough to propel
these cosmozoa to other solar systems. Tramping through space, or riding piggyback
on small grains of dust, these legions of microscopic interstellar emissaries
thus brought the good news of life to the rest of the Galaxy.
Carl Sagan has done a careful analysis of the problem,20
the details of which will not be repeated here. His conclusion is that radiopanspermia
is not a viable theory of the origin of life on Earth. Those microbes ejected
from a stellar system by radiation pressure accumulate a dose of x-rays and
UV three or four orders of magnitude higher than the maximum lethal irradiation
sustain able by even the hardiest terrestrial organisms. Shielding won’t
help: Life-forms large enough not to be killed aren’t ejected by radiation
pressure because they are too heavy.22
The theory that life arose in the ancient swirling gas and
dust clouds of interstellar space and then traversed the cosmos, seeding the
Galaxy with life, may be called cosmospermia. Dr. J. Mayo Greenberg at New York
State University set up a laboratory experiment a few years ago, using tiny
grains of matter the size of space dust and appropriate gases. He found that
many compounds of relatively high molecular weight could be formed under the
influence of ultraviolet radiation. Greenberg evidently believes that a similar
mechanism could lead to the production of grains of a size and composition similar
to that of viruses.
Dr. Sagan has disputed such theories, noting that any hypothetical
extraterrestrial organism of 10-5 cm -- the size of a rabies virus
or the PPLO (the smallest lifeform known) -- would have a replication time on
the order of two hundred million years. There could only have been fifty or
so generations since the Galaxy first formed, insufficient time for natural
selection and evolution to operate.141 It is hard to imagine a smaller
yet viable organism; the replication time for a larger microbe would be even
longer, permitting still fewer generations.
Accidental panspermia is a class of theory typified by the
"Gold Garbage Theory," popularized by Dr. Thomas Gold, a leading astrophysicist
at the Center for Radiophysics and Space Research at Cornell University. The
Garbage Theory was first announced in a paper read before a Los Angeles meeting
of space scientists in late 1958,139 and proposes that Earth may
have been visited by an expedition of advanced ETs who carelessly allowed some
of their native microbiota (picnic basket litter?) to escape. "While this
garbage theory of the origin of life understandably lacks appeal," one
xenologist notes wryly, "we should not exclude it altogether."20
A similar idea is the concept of directed panspermia, which
suggests that organisms were deliberately transmitted to Earth by intelligent
beings on another planet.1283 Advanced civilizations might intentionally
seed sterile worlds, either as a prelude to colonization or perhaps simply to
perpetuate the heritage of life on the home planet as insurance against catastrophe.
Panspermia does not address the phenomenon of abiogenesis
but merely displaces the problem in space and time.* Consequently, panspermia
hypotheses aren’t strictly relevant to the ultimate origin of life in
the universe but simply explain how any particular world might have come to
be inhabited.
* One science fiction story suggests that
life on Earth may have arisen from biota left behind by a careless time traveler
from our planet’s future.636 If any theory begs the question
it is this one!
7.2
Cosmochemical Evolution
The building blocks for life are lying around everywhere.
Great clouds of organic molecules have been discovered drifting
between the stars, presumably formed by various natural processes.1002,2219,2220,2221
Radioastronomers have seen relatively complex compounds hiding deep in inter
stellar space, including methyl alcohol, ethyl alcohol, cyanogen, formaldehyde,
formic acid and ether,1002,2217 and the search is on for amino acids.
Compounds of carbon and hydrogen, particularly cyanogen, methane
and hydrocarbon radicals, are detected on the surfaces of stars.1973,2297
To find the limits of such processes, Dr. John Oró performed an experiment
which simulated a hot stellar plasma. Using a graphite resistance apparatus
and a plasma torch device temperatures from 1500-4000°C were obtained. Methane,
ammonia and water were introduced continuously. The products were condensed
at room temperature and allowed to interact for a few hours before analysis.
Three amino acids appeared -- alanine, glycine, and aspartic acid -- along with
hydrogen cyanide and a host of other organics.1072
There is no doubt that the carbon compounds essential for
the development of Earthly life are ubiquitous. Organics have been detected
on the Moon,2443 other planets,2037,2046 asteroids,2037
and in comets.1973,2222
The carbon chemistry of meteorites is also well-documented.702,2219
The Murcheson rock which fell in Australia on September 28,
1969 contains 2 × 10-7 moles of amino acids per gram of meteorite,
which is more than many desert sands on Earth.521 These amino acids
correspond rather closely to those produced in prebiotic synthesis experiments
performed in the laboratory.225
The Orgueil meteorite contains approximately 7% organic matter,
including hydrocarbons, fatty acids, aromatics, porphyrins, nucleic acid bases,
optically active lipids, and a variety of polymeric material.1075
On the basis of the amounts of carbon compounds detected in various meteorites,
researchers have concluded that these interplanetary wanderers could have brought
as much as 5 × 1010 kg of formaldehyde and 3 × 1011
kg of amino acids to Earth during the first eon of its existence.134
Taken together, these studies of meteorites, comets, planets
and interstellar matter strongly suggest that chemical evolution is a continuing
and commonplace occurrence in all parts of the cosmos. The basic constituents
necessary for the emergence of life are universal. This implies that life should
be widely distributed throughout the Galaxy, wherever conditions are clement,
since the required ingredients of abiogenic processes are abundantly available
everywhere.
7.3
Early Chemical Evolution on Earth
Chemical evolution refers to the period in Earth’s history
during which the chemical components on the surface changed from simple forms
into complex substances from which the first living organisms -- protobionts
-- could develop. The primary investigative tool in abiogenesis research has
been the prebiotic synthesis experiment. Plausible primitive Earth conditions
are arranged in a closed laboratory apparatus, and the changes that take place
are carefully monitored.
The argument has long been made that since no geological record
of the origin of life exists, the course of events leading up to the creative
event is fundamentally unknowable. While most biochemists today would dispute
this supposition, how close to reality are the simulated prebiotic experiments?
It is unnecessary for scientists to heat together water, methane,
ammonia and hydrogen (components of the primitive atmosphere), irradiate the
mess with various forms of energy, and then sit back to wait for a recognizable
lifeform to reach its slimy paw over the edge of the beaker and crawl out onto
the lab desktop. We won’t ever achieve this kind of completeness, because
that takes evolution and the secret to evolution is time.225 (But
it has been seriously suggested that a complete artificial seashore be set up
to test some of the proposed mechanisms in the origin of life.1630)
From chemical equilibria we know the kinds of substances that
had to be floating around in the primitive atmosphere and seas. Protein molecules
ultimately consist of different combinations of only twenty different amino
acids. Nucleic acids are composed of one of five bases, one of two sugars, and
a single type of phosphate group. As Cyril Ponnamperuma of the NASA/Ames Exobiology
Division once remarked: "The alphabet of life is extremely simple; the
wide variety of life observed today may be traced to a mere handful of chemicals."85
Abiogenesis research differs markedly from most other scientific
work, in that an unverifiable historical process is being reconstructed. It
probably is not practical to run through an entire origin of life "from
scratch," so different criteria must be used to evaluate hypotheses. For
instance, postulates must at least be consistent with known astronomical, geophysical,
and biochemical principles insofar as this is possible. And stepwise experiments,
in which only one step of abiogenesis at a time is simulated, are reasonable
if plausible and appropriate prebiotic conditions are maintained.
It is believed that the origin of life may have happened very
fast, certainly less than a billion years521 and possibly less than
a hundred million years.225,305,2160 Most estimates today place the
creative event in the primitive seas, roughly 4.2 to 3.6 eons ago.
7.3.1
Prebiotic Synthesis
For many years it was known that mixtures of carbon dioxide,
ammonia and water vapor would produce small amounts of simple organic chemicals
if energy was supplied. But the results of these experiments were generally
very discouraging and the yields miniscule under these oxidizing conditions.
To originate life in such a poor, thin broth would be well-nigh impossible.
In 1953 a graduate student named Stanley Miller, working under
Nobelist Harold C. Urey at the University of Chicago, constructed an apparatus
to imitate the conditions of the primitive Earth (Figure 7.1). Previous investigators
had always assumed the atmosphere to be oxidizing or neutral. Miller and Urey,
following the suggestions of A. I. Oparin in the Soviet Union and J. B. S. Haldane
in Britain during the 1920’s, took the unprecedented step of devising
a reducing environment instead.2258
Figure 7.1 Miller Apparatus for Prebiotic
Synthesis2315
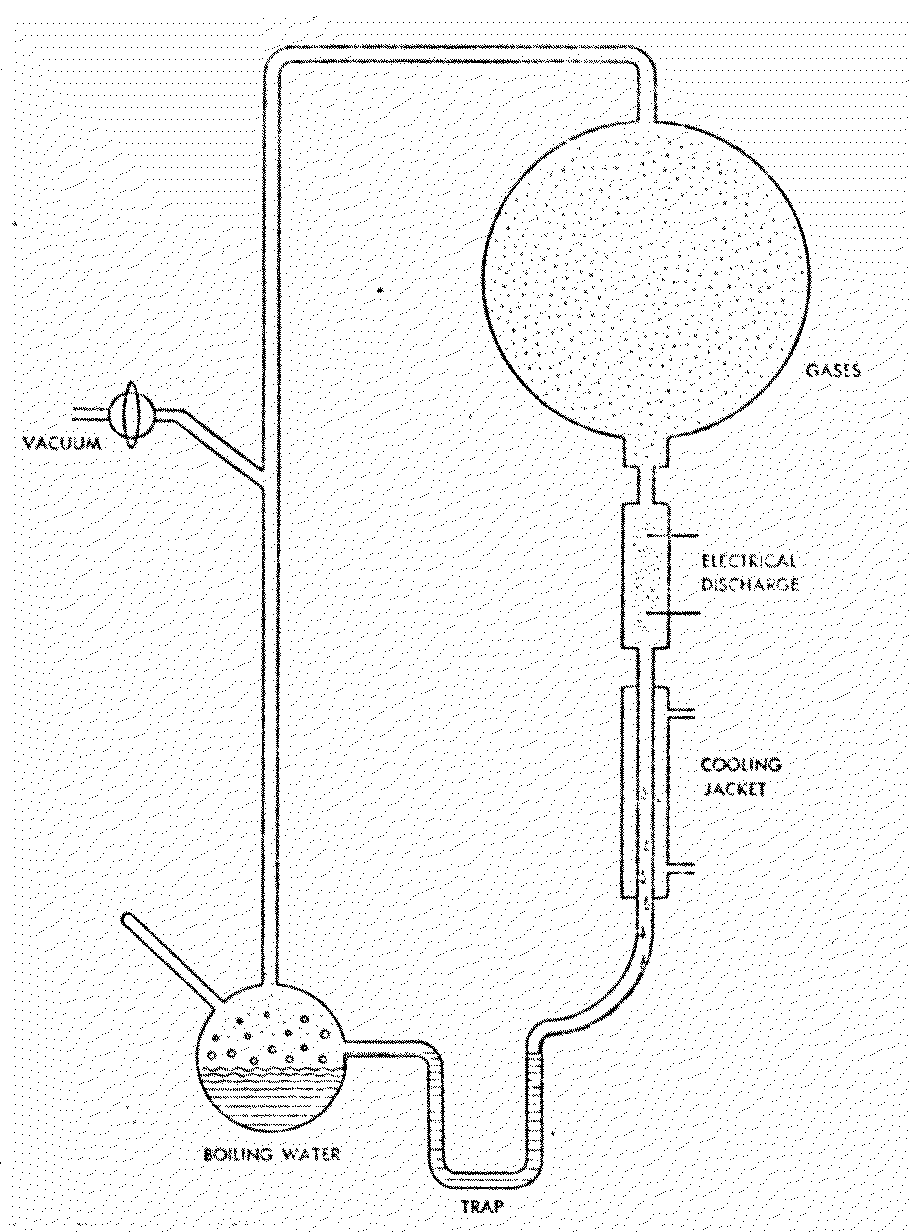
In this schematic of the apparatus
used in Stanley Miller's s historical experiment, a variety of organic
compounds are synthesized as the atmosphere of methane (CH4), ammonia
(NH3), hydrogen (H2) and water vapor (H2O)
is subjected to an electric spark discharge. Circulation is maintained in
the system by the boiling water on one end and the condensing jacket on the
ether.
After one week of continuous
operation, the water was removed and tested by paper chromatography. A great
abundance of amino acids and other organics was detected.
Miller mixed together methane, hydrogen, ammonia and water,
and carefully eliminated all oxygen from the system. This gaseous concoction
was then circulated past an electric spark discharge, followed by a water bath
to simulate the primitive sea. After about one week of continuous operation,
the "ocean" had turned a deep reddish-brown.
The experiment was halted and the contaminated water removed
for analysis. Miller discovered to his amazement and delight that many amino
acids had been produced in surprisingly high yields. Two percent of the total
amount of carbon in the system was converted into glycine alone. Sugars, urea,
and long tarlike polymers too complex to identify were also present in unusually
high concentrations.
Of course, electrical energy was only one of the many sources
of energy available on the primitive Earth (Figure 7.2). In fact, ultraviolet
radiation was probably the principle source: UV would have been able to penetrate
to the surface be cause the protective ozone layer in the upper atmosphere did
not yet exist. A Miller-type experiment using ultraviolet rays and a reducing
atmosphere was performed in 1957 by the German biochemists W. Groth and H. von
Weyssenhoff at the University of Bonn.2307 Their results closely
paralleled those obtained at the University of Chicago half a decade earlier.
Figure 7.2 Prebiotic Chemical Evolution
on the Primitive Earth
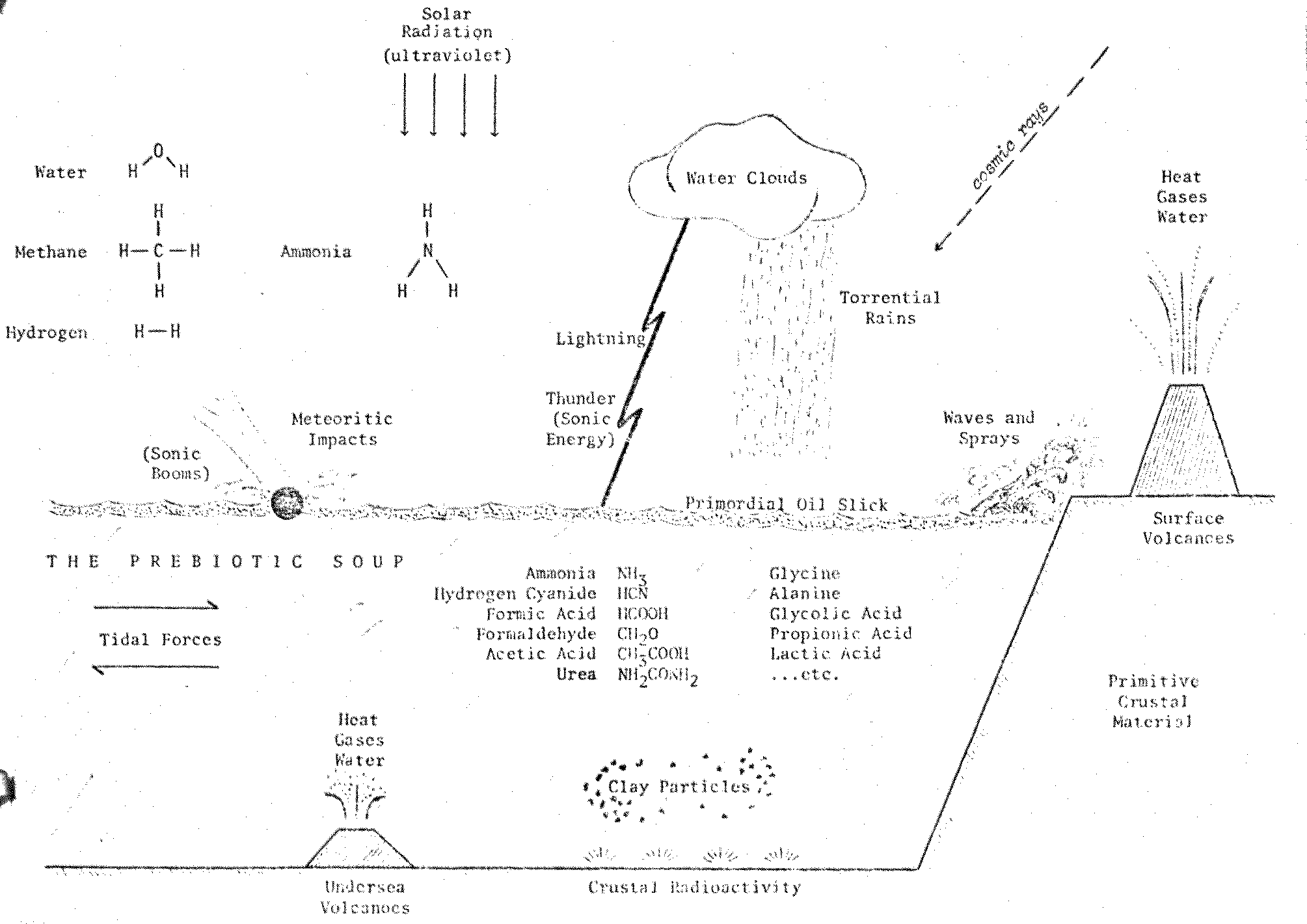
Countless prebiotic simulations have since been achieved which
confirm Miller’s original conclusions. One bibliography, current through
1974, lists more than three thousand papers on the subject.1679 An
exhaustive treatment of all of them is clearly beyond the scope of this book,
but the interested reader in encouraged to dive into the literature (Table 7.1).
|
Table 7.1 Summary of Prebiotic Synthesis Experiments
through 1975
|
|
Year
|
Reactants
|
Energy Source
or Catalyst |
Products
|
Ref. #
|
|
AMINO ACIDS, FATTY ACIDS, AND SIMPLE
ORGANICS |
|
|
1828
|
Ammonium sulfate, potassium cyanate
|
Thermal reaction
|
Urea
|
1586
|
|
1913
|
|
Electrical discharge
|
Glycine
|
2257
|
|
1926
|
CO2, CO, CH4 or H2
|
a-rays
|
Resinous organic material
|
2224
|
|
1937
|
CO + H2O
|
UV
|
Formaldehyde, (HCO)2 (glyoxal)
|
2317,2266
|
|
1951
|
CO2, H2O, Fe++
|
a-rays
|
Formaldehyde, formic, succinic acids
|
2226
|
|
1952
|
formic acid + H2O
|
a-rays (40 MeV)
|
(COOH)2
|
2279
|
|
1953
|
acetic acid + H2O
|
a-rays
|
Malonic, malic, capric acids
|
2278
|
|
1953
|
CH4, NH3, H2, H2O
|
Electrical discharge
|
Glycine, alanine, aspartic & glutamic acids
|
2258
|
|
1954
|
CH2O, H2O, KNO3, ferric
chloride
|
Sunlight
|
Amino acids
|
2261
|
|
1955
|
Ammonium fumarate
|
Heat
|
Aspartic acid, alanine
|
2267
|
|
1955
|
CH4, NH3, H2, H2O
|
Electrical discharge
|
Amino acids, hydroxy acids, urea, HCN
|
2259
|
|
1956
|
CH4, NH3, CO2, H2O
|
|
Amino acids
|
2260
|
|
1957
|
CH4, NH3, H2, CO2,
H2, CO, N2
|
X-rays
|
Amino acids
|
2262
|
|
1957
|
CH4, NH3, H2O
|
UV
|
Simple amino acids, fatty acids
|
2269
|
|
1957
|
Ammonium acetate
|
b-rays
|
Glycine, aspartic acid, diaminosuccinic acid
|
2223
|
|
1957
|
Glycine
|
Heating w/quartz sand
|
Alanine, aspartic acid
|
2264
|
|
1957
|
Ammonium carbonate
|
g-rays
|
Glycine
|
2227
|
|
1959
|
Formaldehyde, hydroxylamine
|
Thermal reaction
|
Glycine, alanine, serine, and others
|
2265
|
|
1960
|
Hydroxy acids ammonia or urea
|
Heat
|
Glycine, alanine, aspartic & glutamic acids
|
2263
|
|
1960
|
CO2, H2O
|
g-rays
|
Formic acid
|
2277
|
|
1961
|
CH4, NH3, H2
|
Accelerated protons
|
Urea, acetone, acetamide |
2316
|
|
1961
|
NH3, HCN, H2O
|
Heat (70 °C)
|
Amino acids
|
2270
|
|
1961
|
CO2, C2H4
|
g-rays, or high pressures
|
Long chain fatty acids (C-40)
|
2276
|
|
1962
|
CH4, NH3, H2
|
b-rays
|
Simple aliphatics incl. amino acids
|
2272
|
|
1963
|
CH4, NH3, H2, H2O
|
Hypersonic shock wave
|
Many unidentified organic compounds
|
2318
|
|
1964
|
CH4, NH3, H2O + silica
|
Heat (850 °C)
|
Amino acids
|
2273
|
|
1964
|
CH4
|
Electrical discharge
|
Higher aromatic hydrocarbons
|
2274
|
|
1966
|
CH4 + silica contact
|
Heat (1000 °C)
|
Higher aromatic hydrocarbons
|
2271
|
|
1966
|
Cyanoacetylene, HCN, NH3, H2O
|
Heat (100 °C)
|
Aspartic acid
|
2275
|
|
1970
|
CH4, C2H6, NH3,
H2O
|
Shock waves ("thunder")
|
Amino acids
|
1664
|
|
1974
|
CH4, NH3, H2, H2O
|
Electrical discharge
|
Amino acids and simple organics
|
521
|
|
SIMPLE SUGARS AND CARBOHYDRATES |
|
|
|
1924
|
Formaldehyde + H2O
|
UV
|
Hexoses, hydroxy acids
|
2280
|
|
1959
|
Monosaccharides
|
g-rays
|
Polysaccharides
|
2310
|
|
1962
|
CH2, Ca(OH)2, CH3CHO,
CH2HCHOHCHO
|
Heat (50 °C)
|
2-deoxyribose, 2-deoxyxylose, etc. |
2281
|
|
1963
|
Formaldehyde + H2O
|
UV
|
Ribose, deoxyribose
|
2243
|
|
1965
|
Glucose
|
Heat (130 °C)
|
Polyglucose
|
2314
|
|
1965
|
Formaldehyde
|
UV
|
Ribose, deoxyribose, other sugars
|
2282
|
|
1965
|
Monosaccharides
|
UV
|
Disaccharides
|
2313
|
|
POLYPEPTIDES, AMINO ACID POLYMERS, PROTEINOIDS |
|
|
|
1954
|
Amino acids
|
Heat
|
Amino acid polymer proteinoids
|
2268
|
|
1954
|
Glycine
|
Electrical discharge
|
Polyglycine
|
2283
|
|
1959
|
Amino acids
|
X-rays, UV, or g-rays
|
Amino acid polymers
|
2310
|
|
1959
|
Hot proteinoid material
|
Heat, slow water cooling
|
Proteinoid microspheres
|
2286
|
|
1960
|
Glycinamide
|
Heat (100 °C)
|
Polyglycine (up to 40-unit strands)
|
2311
|
|
1961
|
Asperine in water
|
Heat
|
Amino acid polymers
|
2285
|
|
1961
|
Glycine
|
Heat (140-160 °C)
|
Polyglycine (up to 18-unit strands)
|
2312
|
|
1964
|
Glycine + Glucose + H2O
|
Heat
|
Amino acid polymers
|
2284
|
|
1964
|
Amino acids
|
Heat + cold quenching
|
Proteinoid microspheres
|
1702
|
|
1969
|
Alkanes, Mg++, PO4=
|
UV
|
n-Hexadecane membranes
|
1415
|
|
1974
|
|
|
Coacervates (a review)
|
1432
|
|
1974
|
Proteinoid
|
|
Higher bonding in proteinoid microspheres
|
1435
|
|
1975
|
HCN, H2O
|
|
Heteropolypeptides
|
1438
|
|
NUCLEIC ACID BASES: PURINES AND PYRIMIDINES |
|
|
|
1926
|
Malic acid, urea, strong mineral acid
|
Thermal reaction
|
Uracil
|
2292
|
|
1960
|
Amino acids
|
|
Purines
|
2289
|
|
1961
|
Malic acid, urea, polyphosphoric acid
|
Heat (100-140 °C)
|
Uracil
|
2244
|
|
1961
|
Amino acids
|
|
Purines
|
303
|
|
1962
|
Urea + AICA
|
Heat
|
Guanine, xanthine
|
2281
|
|
1962
|
HCN, etc.
|
|
Purine intermediates
|
2290
|
|
1963
|
CH4, NH3, H2O
|
b-rays
|
Adenine
|
2237
|
|
1963
|
Urea + CH2CHCN or NH2CH2CH2CN
|
Heat (130 °C)
|
Uracil
|
2293
|
|
1963
|
CH4, NH3, H2
|
b-rays
|
Adenine
|
304
|
|
1963
|
|
Heat
|
Guanine
|
2238
|
|
1964
|
Aspartic acid, glutamic acid 16 others
|
Heat (180 °C)
|
Guanine
|
2239
|
|
1966
|
Urea + AICA
|
Heat
|
Guanine, xanthine
|
2291
|
|
1971
|
|
|
Thymine
|
2246
|
|
1971
|
|
|
Adenine, Guanine, Cytosine
|
2241
|
|
1972
|
|
Zeolite catalyst
|
Purines
|
2247
|
|
NUCLEOTIDES AND POLYNUCLEOTIDES |
|
|
|
1962
|
Nucleotides
|
g-rays
|
Polynucleotides
|
2256
|
|
1963
|
Adenine, ribose, ethyl metaphosphate
|
UV
|
Adenosine triphosphate (ATP)
|
2294
|
|
1965
|
Nucleosides + phosphates
|
Heat (160 °C)
|
Nucleotides
|
2242
|
|
1965
|
Bases + metaphosphate ester
|
|
Polynucleotides
|
2235
|
|
1967
|
Cytidylic acid + polyphosphoric acid
|
|
Cytosine nucleotide
|
2236
|
|
1967
|
Nucleosides + polyphosphoric acid
|
Heat (22 °C)
|
Nucleotides, nucleoside triphosphates
|
2295
|
|
1970
|
Imidazole + cyanamide
|
|
Mononucleotides
|
2252
|
|
1971
|
|
Heat
|
Polynucleotides
|
2251
|
|
1971
|
Cyanamide
|
|
Mononucleotides
|
2253
|
|
1971
|
Adenine nucleotide
|
UV
|
Adenine polynucleotide
|
1628
|
|
1971
|
Cyclonucleoside
|
Heat
|
Polynucleotides
|
1627
|
|
1971
|
Nucleotide
|
Heat
|
Polynucleotides
|
1626
|
|
1971
|
|
|
Cytosine nucleotide
|
2254
|
|
1972
|
Thymine nucleoside
|
|
Thymine nucleotide
|
2245
|
|
3972
|
Ammonium cyanide + water
|
|
Guanine nucleoside
|
2240
|
|
1972
|
|
Apatite catalyst
|
Mononucleotides
|
2250
|
|
1973
|
Cyanamide + AICA
|
|
Mononucleotides
|
2248
|
|
1973
|
Cyanogen + water
|
Apatite catalyst
|
Mononucleotides
|
2249
|
|
1974
|
Nucleotides
|
|
Polynucleotides
|
1435
|
|
1974
|
Nucleosides
|
Heat
|
Oligonucleotides
|
1429
|
|
1975
|
Nucleoside + ammonium oxalate
|
Heat
|
Nucleotide
|
1439
|
Table 7.2 lists the sources of energy believed to be present
during the first eon or so of Earth’s history. Ultraviolet radiation leads
the pack. Carl Sagan and others have completed experiments with UV which seem
to indicate rather high yields for prebiotic amino acids, the building blocks
of proteins. Over the first billion years of chemical evolution on this world
something like a hundred kilograms of amino acids per square centimeter may
have been produced, resulting in a "soup" of about 1% concentration. This is
the approximate consistency of chicken bouillon.
|
Table 7.2 Energy Available for Synthesis of Organic
Compounds on the Primitive Earth
|
|
Source of Energy
|
Energy Available
(joules/meter2/year)
|
|
Solar radiation, all wavelengths |
1.1 × 1010
|
|
Ultraviolet, |
l < 3000 Ang |
1.4 × 108
|
|
l < 2500 Ang |
2.4 × 107
|
|
l < 2000 Ang |
3.6 × 106
|
| |
l < 1500 Ang |
1.5 × 105
|
|
Electrical discharges |
1.7 × 105
|
|
Decay of crustal K-40, 4 eons ago |
1.2 × 105
|
|
(Decay of crustal K-40, today) |
(3.4 × 104)
|
|
Shock waves |
4.6 × 104
|
|
Heat from volcanoes |
5.4 × 103
|
|
Meteoritic impact |
4.2 × 103
|
|
Cosmic rays |
6.3 × 101
|
But ultraviolet radiation is a two-edged sword. While it
may be the most abundant form of energy for molecule building, it is also the
most destructive. Early researchers were concerned that organics would be destroyed
as fast as they were created. Fortunately, the primitive oceans probably turned
opaque like the brownish glop in Miller’s apparatus rather quickly. Vital
chemicals newly synthesized and carried a short distance beneath the surface
of the soup by convection undoubtedly escaped decomposition.
Of the remaining energy sources, electrical discharge was
the most potent. As much as 5-15% of the carbon in a mixture of methane, ammonia
and water may be converted to amino acids and other organics by the energy of
the discharge. Various forms of ionizing radiation give high yields as well.
a particles, b particles, and g rays were common on the surface of the primitive
Earth because of the presence of intense natural radioactive sources in the
crust -- such as potassium-40, thorium-232, and isotopes of uranium.
Volcanic heat was another prebiotic power supply.2368,2380
It has been shown that lava-heated seawater and underwater volcanoes may be
effective in producing biologically important compounds. Heat and sonic energy
would have been released by infalling meteorites -- certainly a significant
factor in the environment of the primitive solar system.1417,2375
In fact, experiments performed recently by Bar-Nun and others have conclusively
demonstrated that as much as 30% of the nitrogen in an ammonia atmosphere can
be converted into amino acids in this manner.315,1664,2375 Torrential
rains have even been suggested as a possible source of energy for prebiotic
synthesis, and experiments have shown that a flask of formaldehyde, allowed
to stand for a few days at room temperature, will produce some simple sugars.
The great lesson appears to be that the exact nature of the
power supply is relatively unimportant. Amino acids, sugars, and other chemical
precursors to life probably arise on any planet possessing an initially reducing
atmosphere and quantities of hydrogen, carbon, nitrogen and oxygen in gaseous
reduced form -- regardless of the particular source, or sources, of energy available.*
* Other factors may also be important. For
instance, early-type stars (F) are more likely to emit ultraviolet radiation
in copious quantities than are late-type stars (K, M). The speed of chemical
evolution in primitive planetary environments may actually slow as we move from
class F through classes G to K stars among habitable solar systems.
7.4
Proteins and Cells
The cell is the fundamental biological unit and a common denominator
among all terrestrial lifeforms. Living things on this planet are made up of
cells which vary in size from less than one micron to several centimeters in
diameter. While the simplest organisms are unicellular, the typical human is
an ambulatory assemblage of from fifty to a hundred trillion (1014)
individual cells.
The cellular construction of Earth life is remarkably uniform:
Similar water content, similar kinds of proteins, similar lipids and so forth.
All have at least one membrane, perhaps no more than 100 Angstroms thick, which
protects the inner workings from the harsh vagaries of the external environment.
The first protobionts undoubtedly had no such complex organizational qualities.
But how can structure arise in the first place?
It has been shown by Ilya Prigogine that thermodynamic chemical
systems may develop certain states wherein some of the chemical constituents
have periodic, oscillating values.2230 A biologist, J. Pringle, has
demonstrated that initially homogeneous systems can undergo a progressive change,
leading to the appearance of "spatial heterogeneity."2231,2232
That is, structure can arise spontaneously. These two treatments of the problem
of organization suggest that mechanisms may exist for collecting material into
small, localized concentrations, perhaps leading to ordered structures we would
recognize as cells.2368
But to build cells, we must have protein. Protein is the most
fundamental construction material, used in building cell walls, enzymes, and
so forth. To make proteins, there are two requirements.
First, we need an abundance of amino acids. From our discussion
above, we see that this is virtually inevitable on any normal world possessing
at least small aqueous oceans and a primitive hydrogenous atmosphere.
Second, there must be some way to hook up a long string of
amino acids into a polymer of protein. Polymerization (linking together) of
amino acids leads to the production of protein, which can then be used for cell-building.*
It is true that even dilute primordial soups can coagulate into gelatinous masses.
But such conditions are far from ideal. In all likelihood, most prebiotic syntheses
probably took place in local regions of increased concentration. The efficient
construction of amino acid polymers undoubtedly occurred elsewhere than in the
open seas.
Numerous concentration mechanisms have been proposed which
might conceivably lead to the creation of small pockets of more potent broth.
The simplest method is evaporation. Primordial soup, caught in a narrow, shallow
lagoon, would slowly thicken as the water that held the components in solution
evaporated away.85 As suggested by Miller and Orgel, similar effects
result from slowly freezing the solution in the lagoon: The solvent freezes
out in the pure form first, leaving the solute concentrated in ever-smaller
quantities of solvent.521
A combination of air-water and water-solid interfaces provides
mechanical consolidation of suspended matter, as evidenced by the accumulation
of scums and oil slicks near coastlines.1667 Another possibility
is that organic compounds may have been trapped on solid surfaces such as aluminum
silicate clays, quartz, and other minerals which allow polymerization reactions
to proceed.1430,2381
To date, however, there is really only one proven method which
yields polymers of amino acids under plausible prebiotic conditions. Dr. Sidney
W. Fox at the University of Miami has obtained long-chain molecules with the
following essential properties:
1. They contain all amino acids common in contemporary
terrestrial organisms.
2. They have high molecular weights (the chains are relatively
long).
3. They are "active" because they interact in
the catalytic or rate-enhancing sense. (This anticipates metabolic activities
mediated by enzymes -- which are also proteins.
4. They are as heterogeneous as contemporary proteins.
5. They yield "organized units" upon contact
with water which have many properties in common with modern cells.1625
Dr. Fox calls his substances "proteinoids," because
they greatly resemble living protein polymers. His method for producing them
is quite simple. A mixture of amino acids is cooked at 120-170 °C for a
few hours, and substantial yields (10-40%) of polymeric material are obtained.
Fox decided to test his method under more realistic field
conditions. He secured a large piece of lava from the site of an Hawaiian volcano.1702
The temperature of the rock was raised to 170 °C, and the appropriate amino
acids seated in a small depression at the top. Heating continued for several
hours, after which the lava was washed off with a small spray of sterilized
boiling salty water -- as might have occurred naturally near a volcanic shoreline
in ancient times. Proteinoid polymers were formed, but there was more! To Dr.
Fox’s surprise, billions of tiny "microspheres" appeared in
the wash water: spherical, microscopic particles of uniform diameter bearing
a striking resemblance to living cells (Figure 7.3).
Figure 7.3 Possible Model Protobionts:
Thermal Proteinoid Microspheres1625
|
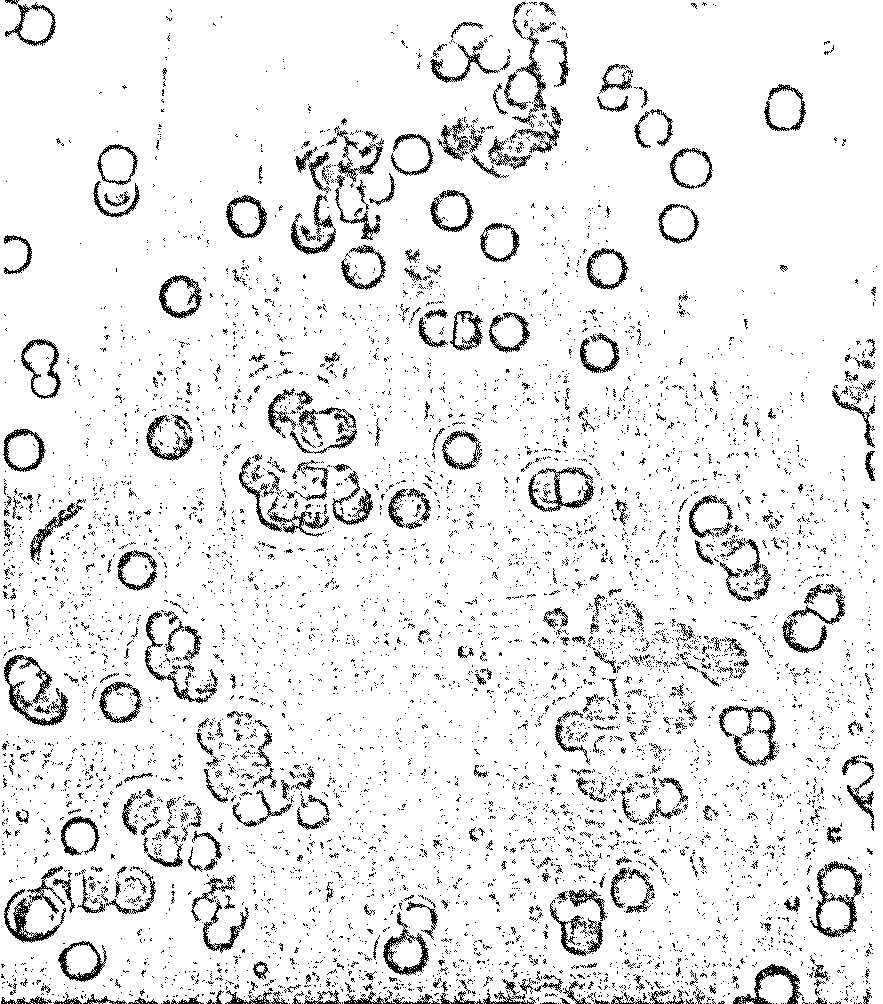
|
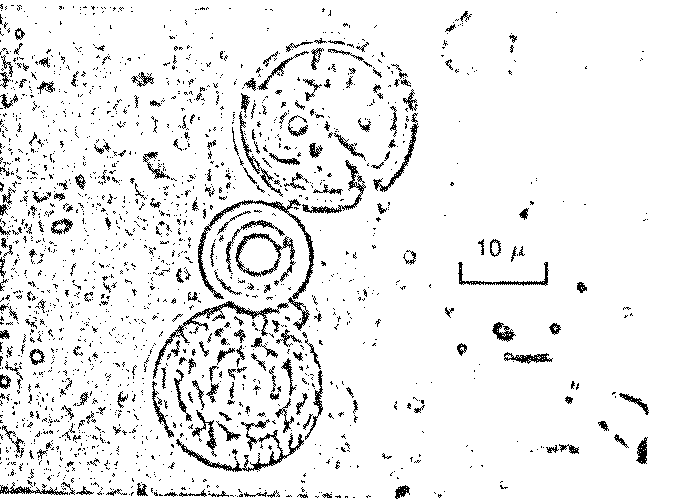
Structured thermal proteinoid
microspheres.
|
These proteinoid microspheres
were produced by slowly cooling a hot, clear solution of thermally
polymerized amino acids.
|
|
|
(At right) Various stages of binary
fission of proteinoid microsphere "protocells"
|
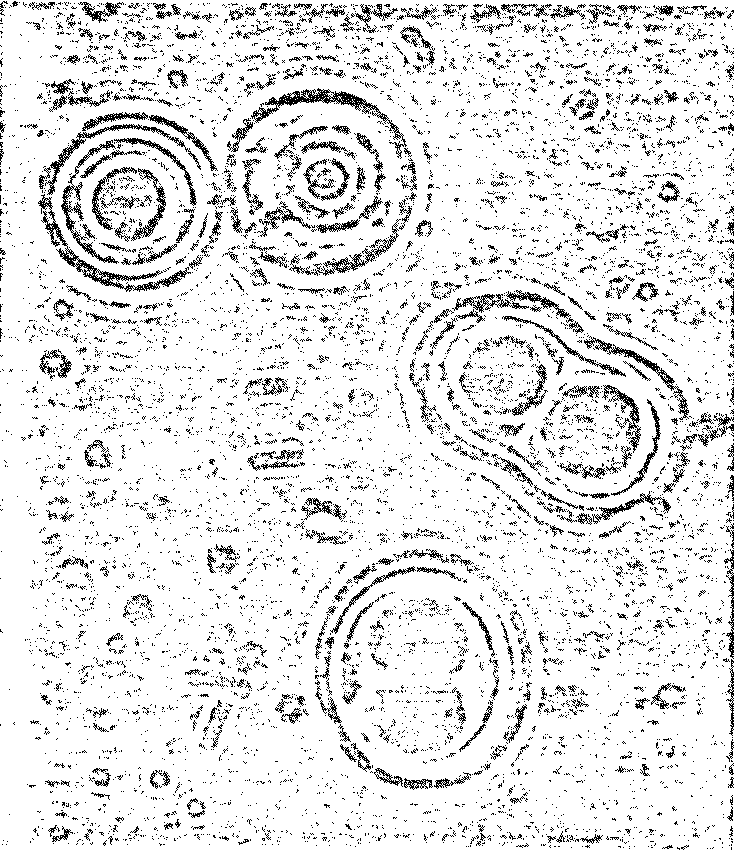
|
|
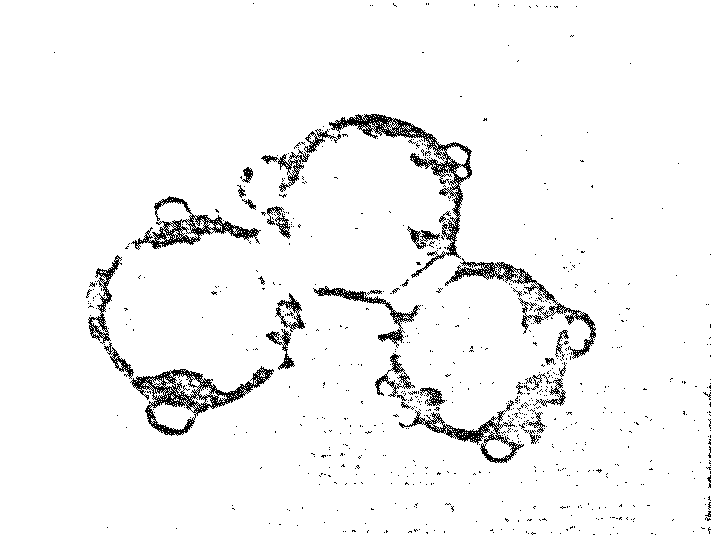
|
|
(Above) Parent microspheres spout
buds.
|
|
(At right) Second-generation
laid on second generation microsphere. After the bud grows to maturity,
it sprouts its own new buds. Is this a form of incipient reproductive
capability?
|
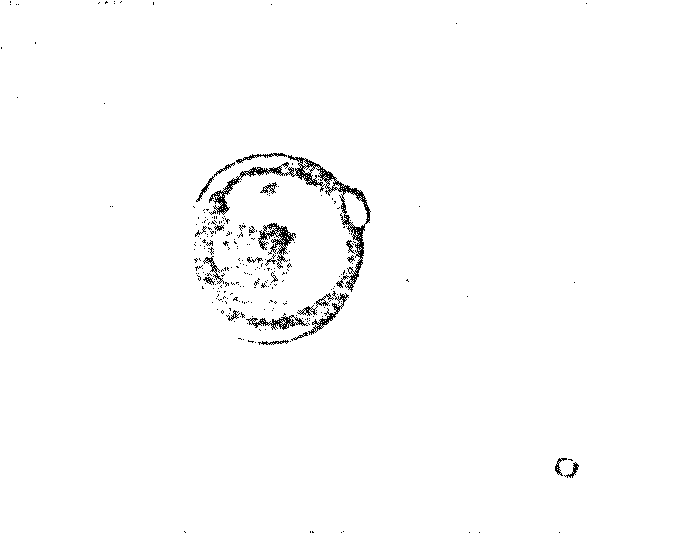
|
The Miami scientist presented a scenario for the origin of
microsphere protocells in prebiotic times: (1) Hot lava meets soup; (2) water
boils away, leaving sticky brown goop on lava; (3) contact with water (rain,
sea spray, etc.) causes proteinoids to assemble themselves; and (4) microspheres
are washed back into the soup.
These initial experiments were completed nearly two decades
ago,1702 and since that time Fox and his colleagues have refined
their methods and perfected their theories on the origin of cells and life.
Protein-like materials are now produced with molecular weights ranging from
3000 to 10,000 under plausible primitive Earth conditions.2371 And
it has been shown that a primitive “cell” with most of the attributes
of life can arise spontaneously in a very brief period of time.
Detailed studies of microspheres have confirmed the researchers’
initial optimism. What makes these spherules so unique is their “active”
nature. Dr. Fox has observed and recorded the following characteristic behavior
of his proteinoid microspheres under various chemical and physical conditions:
1. Spherical shape -- 0.5 to 7.0 microns, uniformly.
2. Single-walled membranes (like plants) and double-walled
membranes (like animals).
3. Simulation of osmosis -- microspheres swell and shrink
in response to changes in the chemical environment.
4. Selectivity of diffusion -- microspheres possess semipermeable
membranes analogous to those of living cells. For instance, in one case Fox
discovered that polysaccharides were selectively retained under conditions
in which monosaccharides diffused freely through the microsphere walls. (Polynucleotides
and other organics are also absorbed from aqueous solution.1624)
5. Cleavage -- a kind of binary fission of a single “cell”
has been observed in acidic proteinoid microspheres.
6. Motility -- the microspheres, when viewed under a microscope,
move non-randomly in preferred directions under certain special conditions.
The addition of ATP appears to enhance the movement.
7. Budding -- buds appear spontaneously on proteinoid
microspheres allowed to stand undisturbed in their mother liquor.
8. Growth by accretion -- buds which have been liberated
by mild heating or electric shock will swell by diffusion to the same approximate
size as the “parent” cell.
9. Proliferation through budding -- second generation
budding has frequently been observed on buds that grew to the size of normal
microspheres. The buds are apparently engaging in a kind of “reproduction.”
10. Formation of junctions -- microspheres are capable
of approaching one another and physically attaching together in a more or
less permanent fashion.
11. Transmission of information -- when two spheres have
joined, small proteinoid microparticles within the larger sphere are observed
to pass through the junctions. The whole process is highly suggestive of microbial
conjugation.
12. Stability -- the activity of the proteinoids does
not diminish with storage over a period of 5-10 years.2370
The best-known of all physical cell models prior to the discovery
of proteinoids was the coacervates, thoroughly researched by the Soviet biochemist
A. I. Oparin, the Dutch biochemist H. G. B. de Jong, and others. Coacervates
are produced by combining solutions of oppositely charged colloids such as gelatin
or histone with gum arabic. When solutions of the two substances are commingled,
they interact to yield clusters of microscopic structures having the appearance
of tiny liquid droplets. Coacervates have many interesting properties from the
point of view of the origin of life.
For instance, after these uniform spherules have aggregated,
they are able to absorb various simple organic molecules from the external medium
(sugars, dyes, etc.). However, Oparin has admitted that this process quickly
leads to static equilibrium, and the coacervate "protocell" then becomes
a passive system, unstable and prone to break-up upon standing. Another property
of coacervates is their ability to convert certain chemical monomers to polymers
after diffusion through the "cell" wall, although it is generally
recognized that the dynamic behavior of these droplets is fairly limited.
There is another reason why coacervates,1432 sulphobes,1625
"biphasic vesicles,"1630 and many other prospective pseudocells1211,1415
do not compare favorably with Dr. Sidney Fox’s microspheres as model protocells.
Coacervate droplets are formed from polymers which themselves were synthesized
by living organisms. The gum arabic used to manufacture Oparin’s droplets
was not produced abiogenetically, nor is it at all clear how this might be done.
The great advantage of the microspheres is that they are the direct product
of single, simple amino acids -- amino acids that must have been common on the
shores and seas of the primitive Earth eons ago.
Of course, no biologist today would claim that proteinoid
microspheres are alive in the sense of representing the first protocell. And
yet, to the extent that they self-organize, accumulate information from their
surroundings, and exhibit both structure and behavior, they are certainly near
the borderline of life.
* We will not discuss here the significance
of molecular optical activity. The curious reader is referred to Sagan,20
Jackson and Moore,47 Glasstone,72 Gabel and Ponnamperuma,315
Miller and Orgel,521 Ulbricht,1445 Hochstim,1446
Bonner et al,1447 Wald,1665 and Walker.2382
7.5
Nucleic Acids and DNA
In the previous section it was mentioned that there are two
requirements for the production of proteins. First, there must be amino acids,
and second, there must be a way to hook them together to form polymers.
There is, however, a third requirement for the origin of living
systems on Earth. It will be recalled from the discussion of the definition
of life that "it is the business of life to accumulate information and
complexity." Let us consider this mandate in view of the problem of building
proteins.
To abiogenetically produce a living system, that system must
be capable of accumulating information and order from its environment. The proteins
constructed by a cell must have the proper architecture for whatever job needs
to be done. So our third requirement may be stated: There must be a way to hook
the amino acids together in the correct sequence. Any old proteins will not
do -- they must be the right ones.
There exist simple chemical techniques to achieve this kind
of ordering. One common example is called "autocatalysis" by chemists.
Autocatalysis is a way for a process to catalyze its own production. Once a
tiny bit of it has been produced, that bit catalyses the rate of reaction to
yield still more, and faster.
Aside from this simple selective feedback effect, the development
of molecular self-replication was probably the most critical single event in
the origin of life on Earth. The origin of replication and the genetic code,
as opposed to the origin of proteins and cells, allowed natural selection to
begin to operate on stored information. And once evolution begins, selective
advantages of superior membranes and of multicellular colonies can be expressed
in the form of increased organismal complexity.
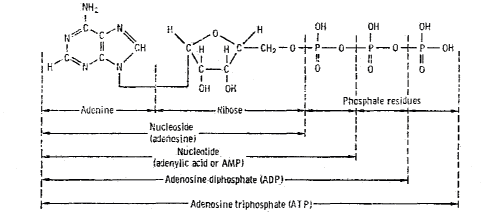
DNA -- the primary information-carrying molecule used by all
lifeforms on this planet -- is a polymeric nucleic acid (Figure 7.4). We’ve
already seen how easy it is to get amino acids and their polymers. But what
about nucleic acids? Can they be demonstrated in prebiotic synthesis experiments,
along with their polymers?
Figure 7.4 The Role of Nucleic Acids
in Terrestrial Biochemistry
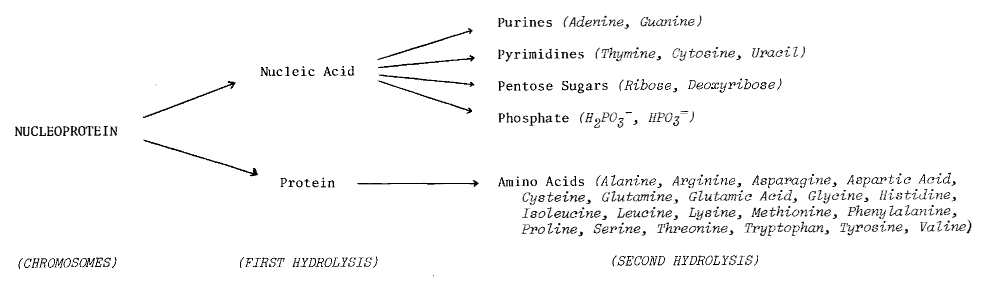
Chemical Structure of Nucleic Acid (from Glasstone72)
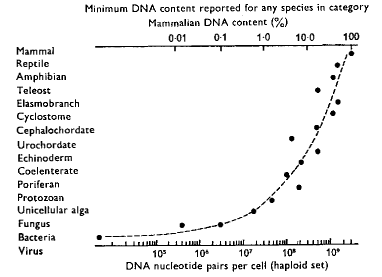
DNA Content (figure from Britten and Davidson2568,
in Kohne1654)
In 1963, Dr. Cyril Ponnamperuma managed to synthesize adenine
(one of the two most important nucleic acid purine bases) under simulated primitive
Earth conditions. The NASA scientist and his three colleagues used a Miller-type
apparatus, and began their synthesis with nothing more than methane, ammonia
and water in the system. The mixture was bombarded with energetic electrons,
and about 0.01% of the carbon in the methane was converted into adenine.304
This is highly significant because adenine is useful, not only for making DNA,
but also RNA, ATP, ADP, FAD, and a host of other critical life-molecules.
In a related experiment two years later, Dr. John Oró
of the University of Houston and A. P. Kimball produced adenine is a closed
reaction system which included ammonia, water, and hydrogen cyanide. Heat was
supplied as the energy source, and this time the production of the purine base
rose to 0.5% of the available carbon.303 This value was observed
over a wide range of chemical conditions, indicating the relative ease with
which this complex molecule must arise in a plausible prebiotic environment.
The synthesis of the other important purine, guanine, has also been convincingly
demonstrated.
There have been various attempts to fabricate the three major
varieties of pyrimidine bases which are also necessary in the production of
nucleic acids. However, the appearance of these substances under conditions
similar to the primitive Earth has not been investigated as thoroughly as the
purines.
One experiment that yields a hefty 20% of cytosine requires
a three-step process involving methane and nitrogen initially to create a cyanoacetylene
intermediate, which then goes on to produce the pyrimidine when combined with
cyanate ion. Uracil, another pyrimidine, is obtained in very good yield by the
direct hydrolysis of cytosine -- a prebiotically reasonable reaction. All the
pyrimidines have been synthesized in environments at least arguably analogous
to that of the early Earth.
Prebiotic assembly of purines and pyrimidines into full-fledged
nucleotides has proven more difficult, and intensive investigations are now
underway to determine and eliminate the problem. The main obstacle to success
seems to be the formidable complexity of the nucleotide molecules themselves.
While bases and sugars are relatively easy to produce, combining them together
is a much harder task.
Nevertheless, demonstrations of nucleotide synthesis under
geologically plausible constraints have been made. One such technique involves
the use of a mediating mineral called apatite, which contains phosphates and
oxalate ion, in an "evaporating pond" scenario.
We are not quite home yet. Just as amino acids needed polymerization
to become protein, so must nucleotides by polymerized into DNA. What progress
has been made in the prebiotic synthesis of polynucleotides?
The experimental record is admittedly spotty. When adenine
nucleotides were heated in the presence of polyphosphate for 18 hours at 55
°C, adenine polynucleotide polymers were obtained ranging from 20-30 nucleotides
per chain. However, in the words of the experimenter, "the concentration
of the reactants had to be as high as possible when the formation of high polymeric
material was desired."1625
That is, unless quite artificial conditions were contrived,
the adenine nucleotides could not be forged into very long chains. In another
experiment, solutions of adenine nucleotide were irradiated with UV light. Long
chains were again obtained, but only when extraordinarily high concentrations
of polyphosphate were maintained.1628 Under similarly unrealistic
conditions, uracil polynucleotides with chain lengths ranging from 10-50 units
have been found.1626,1627
One good experiment has been performed by John Oró
and E. Stephen-Sherwood, using a plausible "evaporating lakebed" scenario
and temperatures from 60-80 °C. Uracil two-unit chains were formed with
a yield of 23%, and three-unit segments with a 12% yield. Cytosine polynucleotide
chains were obtained by these experimenters with up to six nucleotides in straight-line
linkages. Thymine polynucleotides 2-12 units long were produced when an unreasonable
chemical environment was used; with more closely matched prebiotic conditions,
five-unit chains were obtained in yields of 1% or less.1429
The polymerization of some nucleotides has proven unexpectedly
difficult, partly because of the inevitable formation of unnatural side chains
and partly because the reaction just doesn’t seem to want to go. Various
solutions to these problems have been suggested. For instance, there are enzymes
-- ordinary proteins -- that are capable of catalyzing these polymerization
reactions with ease. These enzymes, or enzymes like them, could have arisen
by nonbiological means. If this is the case, claims one researcher, "such
catalysts may have been responsible for the first polymerization of nucleotides
on the primitive Earth."72
So at present, here is where we stand. Purines and pyrimidines
are comparatively simple to manufacture abiogenetically. The assembly of nucleotides
has also met with some limited success, but to date it has proven difficult
to synthesize more than six-unit polymeric chains in a prebiotically plausible
way.2370
Can these short strands alone make a stab at primitive replication?
Dr. Leslie Orgel at the Salk Institute in San Diego, California, mixed up a
solution of nucleic acids that might be considered prebiotically reasonable.
He then placed some of the six-nucleotide polymers in his specially-enriched
"soup." The short-chain DNA polymers correctly replicated themselves
once out of every ten tries.
7.6
Early Biological Systems
Thus far we have concentrated on the parallel development
of polymeric amino acids (proteins) and polymeric nucleotides (DNA). We’ve
seen that Dr. Sidney Fox’s proteinoid microspheres exhibit many properties
which are strikingly similar to those displayed by contemporary living cells.
We’ve also seen that Dr. Leslie Orgel has succeeded in demonstrating accurate,
if erratic, replication in primitive polynucleotides. And yet, despite these
remarkable achievements, the great final question remains untackled: How and
when did the first living organism arise?*
The answer is as unsatisfying as it is precise: No one knows.
The arguments on this score smack of the "chicken-or-the-egg" controversy.
It is unknown at present if proteins and protocells came first, to be followed
later by replicative nucleic acids, or whether the nucleic acids were first,
and from them the cells later spawned.
It has been fairly clearly demonstrated that life as we know
it could not have arisen if either one or the other was wholly absent.521
Organisms lacking nucleic acids would have no means of achieving genetic continuity
and evolutionary progression, while organisms without proteins would find themselves
severely limited in their ability to utilize the chemicals in their environment.
Some manner of coevolution seems to be indicated.
One theory holds that nucleic acids evolved some kind of boundary
layer, a proteinous skin to protect themselves from their surroundings -- the
so-called "naked gene" theory. When this invention inhibited or prevented
reproduction, the parent nucleic acid molecule became extinct. When the new
boundary layer served to protect the DNA without interfering with replication,
these were the "protobionts" which survived.
There is some experimental evidence to support the view that
polynucleotides might be able to influence protein synthesis directly.1431,2255
To do this, they must cause a selective linear organization of amino acids,
and must facilitate amino acid polymerization.1444 Unfortunately,
other studies have shown that the interaction between polynucleotides with individual
(monomeric) amino acids is relatively weak.1248
More convincing, perhaps, is the idea that cells were first.
Self-assembly in molecular structures has been known for many decades, and experimental
evidence to date favors the easy synthesis of proteins in comparison to polynucleotides.1634
Sidney Fox has remarked that the sequence:
protoprotein —> protocell —> nucleic-acid-coded
contemporary cell
is the most valid evolutionary sequence because it proceeds
from the simple to the more complex.1625
The primitive protocell, as modeled by the proteinoid microspheres,
could have exhibited many of the properties customarily regarded as belonging
only to "living" things. Under Fox’s theory, the cell would
have developed nucleic acids to serve its ends, rather than the other way around.
One final piece of evidence seems to argue for the primacy
of cells. In 1974, Dr. Fox and his colleagues published some experimental findings
on micro-spheres which seem to imply that the proteinoid protocell can do everything
optimistically predicted for it. The abstract of the paper reads, in part:
Proteinoid microspheres of appropriate sorts promote the
conversion of ATP to adenine dinucleotide and adenine trinucleotide. When
viewed in a context with the origin and properties of proteinoid microspheres,
these results model the origin from a protocell of a more contemporary type
of cell able to synthesize its own polyamino acids and polynucleotides.1435
(emphasis added)
We’ve seen that scientists have discovered a relatively
smooth chain of synthesis from the stuff of stars to the stuff of life. On the
basis of pre biotic experiments performed to date, it is probable that most
of the organic molecules of life with a molecular weight less than 1000 spontaneously
appeared in significant quantities during the early years of our world. While
a number of problems remain, most indications are that the origin and development
of life on Earth had a certain inevitability about it.
From the simplest compounds present when our planet first
congealed about 4.6 eons ago, to the first viable protobiont some half a billion
years later, the patterns of development and the upward march of complexity
seem unavoidable. Only the most general conditions must be needed for carbon-based
life to arise: A body of water, a primitive reducing atmosphere, some source
of energy, and lots of time. Life, in Soviet Academician Oparin’s own
words, is "an obligatory result of the general growth of the universe."2297
Even now we humans just begin to suspect the truth: The universe
is not ours alone to keep.
* There are countless side issues that cry
out to be discussed at this point, but which unfortunately can be given only
a passing nod. First of all, there is the absolutely fascinating question of
the genetic code. As is well-known, genetic information is written on the DNA
strand in short, three-nucleotide "words" called codons. By properly
reading these encoded blueprints, a cell can construct exactly the right protein
molecules.
For instance, a series of three adenine nucleotides
in a codon tells the cellular machinery to use one molecule of an amino acid
called lysine at that location. Three guanines in a row means that a molecule
of the amino acid glycine should be used. One by one, the codons tell which
amino acid to use and in what order, and proteins are built up in precisely
the right way.
What is the origin of this marvelous code?1064,1444,2383
Is a three-nucleotide codon somehow optimal,1777 or would four have
been more evolutionarily efficient?1064,1066 Why not the simplicity
of only two? And what determined the rules of the coding itself? Three guanines
mean glycine to a virus, a dandelion, or a human. Is the code somehow efficiency-maximized
or error-minimized?1065 (It appears to be!1066,2378)
What is the origin of chromosomes,2301
and the true purpose of genes?2322 These are important questions
for xenobiologists to be asking, because the universality of our genetic mechanisms
will determine the limits of variation that can be expected in alien biochemistries.
For xenologists, of course, there are far
more fundamental issues that must be raised. For example, why must genetic information
be stored digitally in a linear sequence of monomer units? Could not some form
of analog system serve? What of the possibility of genetic systems whose information
was stored, replicated and transcribed in a planar fashion rather than linearly?1777
Dr. Francis Crick has pointed out that on
Earth, DNA is used for "replication" and proteins are used solely
for "expression" or "action." Is it possible, he asks, "to
devise a system in which one molecule does both jobs, or are there perhaps strong
arguments, from systems analysis, which might suggest that to divide the job
into two gives a great advantage?"22 Others have echoed this
idea.521
Similarly, Michael Arbib of the University
of Massachusetts at Amherst questions "whether it is necessary for any
lifeform to have a genotype distinct from a phenotype; in other words, whether
we have to have a program to direct growth and change, or whether in fact the
organism might be able to reproduce itself as a whole."85 Crick
seems to agree,, suggesting that it might be possible to "design a system
which was based on the inheritance of acquired characteristics."22
(At least one science fiction story has been written along these lines.2216)
Arbib also wonders: "One might imagine some planet whose beings reproduce
by xerography with no gene required!"85 The possibility of inheritance
without genes has been suggested before,1178 although in a different
context. (A
general review
of replication was published in 2004 by Freitas amd Merkle.)
And we must take care not to be guilty of
"nucleic acid chauvinism." We are familiar with only one molecular
replicating system, but there is no reason why others should not be possible.
Gordon Allen writes: "Life on other planets need not be based on nucleic
acids or proteins if their catalytic functions can be otherwise provided."1591
Dr. Alexander Rich at MIT also suspects that
the functions of Earthly nucleic acid are not unique. Rich believes that "other
molecules could be used to form other polymers which could be used as information
carriers for living systems." Later, he elaborates:
I think it would be amusing to make a chemical
system of complementary polymers based on monomers that are not nucleic acid
derivatives, simply to demonstrate that it can be done. In about ten years’
time, I think we will have a well-developed field of synthetic polymeric information
carriers that will give us a great deal of insight into our own terrestrial
system. That another system is possible might have relevance, if not to biology
on this planet then perhaps to another.1587,1632
Clearly, a search should be made for non-nucleic
acid self-replicating molecules. Exotic systems based on silicon, boron, or
nitrogen-phosphorus chemistries are possible: Specialists in these fields expect
an abundance of compounds comparable to that of carbon chemistry.1777
But we must not anticipate the subject matter of the next
chapter.
Back to Contents









