|

by Joshua Sokol
13 August 2016
from
Sci-Hub Website
|
Joshua Sokol is a
science writer based in Cambridge, Massachusetts |
Ocean worlds,
the search for life
in the solar system's other
seas.
Our best chance to find alien life lives
in the vast oceans inside the
icy moons of Saturn and Jupiter
and we don't have to leave
Earth
to start looking for.
Oceans inside distant icy moons
are the best prospects for
finding
life beyond Earth.
Suddenly, out of darkness, a ghostly
city of gnarled white towers looms over the submersible.
As the sub approaches to scrape a sample
from them, crew-member Kevin Hand spots something
otherworldly:
a translucent, spaceship-like
creature, its iridescent cilia pulsing gently as it passes
through the rover's headlights.
This is not a dispatch from an alien
world, but it could be.
Hand is a planetary scientist at NASA's
Jet Propulsion Lab in Pasadena, California, and one of a select few
to have visited the carbonate chimneys of the Lost City at the
bottom of the Atlantic Ocean.
It is the site of an extraordinary ecosystem - one that Hand
suspects might be replicated on icy moons orbiting distant gas
giants.
"In my head, I was saying to myself:
this is what it might look like," he says.
Jupiter's moon
Europa, and
Enceladus,
which orbits Saturn, both have vast oceans secreted beneath their
frozen outer shells.
As such, many astrobiologists consider them our best bet in the
search for life beyond Earth. NASA is plotting life-finding missions
there. But we don't have to wait to dip our toes in extraterrestrial
waters.
Having explored extreme ecosystems on our own ocean floor - places
like Lost City, where life is fuelled by nothing more than the
reaction between rock and water - we know what to look for.
Now the race is on to spot signs of
similar geochemical rumblings on Europa and Enceladus, and so
discover whether we truly are alone in the solar system.
"Follow the water" has long been the mantra in the search for life,
and for good reason:
every known organism needs water to
survive.
Most prospecting has been done
on Mars,
but the Red Planet's water is either long gone or locked in the
ground as ice.
These days, even Mars buffs would
struggle to deny that the best prospects for finding living
extraterrestrials lie further from Earth.
It might seem odd to search for liquid water in places far from the
sun's warmth. And yet it looks as if there are sloshing oceans
beneath the surfaces of Europa and Enceladus, thanks to tidal
flexing as a result of their eccentric orbits.
As the gravity of their host planets
pushes and pulls at the moons' interiors, they warm from the inside
out - and that heating is enough to maintain a layer of liquid
between their rocky mantles and icy crusts.
The first hints of Europa's concealed sea came from the
Voyager
probes, which explored Jupiter back in the 1970s. Voyager II spotted cracks in Europa's
icy surface crust, suggesting active processes below.
When the
Galileo spacecraft returned in the 1990s, it saw another clue:
Jupiter's magnetic field lines were bent around Europa, indicating
the presence of a secondary field.
The best explanation is the presence of
a global vat of electrically conductive fluid, and seawater fits the
bill.
We now think this ice-enclosed ocean reaches down 100
kilometers. If so, it contains enough salty water to fill Earth's
ocean basins roughly twice over.
The case for a sea on Enceladus washed in more recently. In 2005,
the
Cassini probe showed that the moon leaves a distinct impression
in Saturn's magnetic field, indicating the presence of something
that can interact with it.
That turned out to be an
astrobiologist's fantasy:
a plume of ice particles and water vapor
shooting into space through cracks near Enceladus's south pole.
Cassini has since flown through these plumes several times.
First its instruments revealed the
presence of organic compounds. They seemed to be coming from a
liquid reservoir - and the particles collected from lowest part of
the plumes were rich in salt, indicative of an ocean beneath.
Cassini detected ammonia, too, which acts as an antifreeze to keep
water flowing even at low temperatures.
All the signs suggested this was a sea
of liquid water, stocked with at least some of the building blocks
of life.
Treasure
plumes
"After decades of scratching around
Mars to find any organics at all, this was an embarrassment of
riches," says Chris McKay, an astrobiologist at NASA's Ames
Research Center in Moffett Field, California.
The treasures kept coming.
In March 2015, Cassini scientists
detected silicate grains in the plumes - particles that most likely
formed in reactions at hydrothermal vents. By September,
measurements of how Enceladus's outer crust slips and slides had
convinced them that it contains a global ocean between 26 and 31
kilometers in depth.
That's a paddling pool compared with
Europa's, but way deeper than Earth's oceans.

Enceladus’s plumes
have got astrobiologists excited
NASA/JPL-Caltech/Space Science Institute
So when do we visit?
NASA has already selected instruments
for a new mission to Europa, set for launch in June 2022. It will
feature a magnetometer to probe the ocean's saltiness and
ice-penetrating radar to show where solid shell meets liquid water.
It might even include a Lander to fish
for amino acids, the building blocks of the proteins used by every
living thing on Earth.
"What could be bending
Jupiter's magnetic field around
Europa?
Seawater fits the bill"
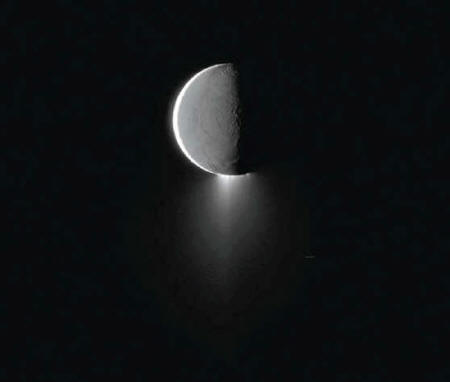

Two of
Saturn's moons may host life:
Enceladus (with its
geysers) and Titan.
The space agency has also invited
proposals for a trip to Enceladus.
One option is the
Enceladus Life
Finder, a probe that will sample plumes using instruments
capable of detecting larger molecules and more accurately
distinguishing between chemical signatures.
Other plans have even
suggested carrying samples back to Earth for analysis.
With any luck, NASA probes will be arriving at these ocean worlds by
the twilight years of the 2020s. Until then we just have to sit
tight, daydreaming about what fresh wonders we might find once we
get there. Or do we?
In fact, there is plenty we can do in the meantime to plumb Europa
and Enceladus's hidden depths. We can survey their surfaces using
ground-based telescopes, gawping at the fissures where water might
bubble through and leave telltale deposits from the oceans beneath.
We can model the geophysics that keeps
them liquid so far from the sun, and may generate conditions that
could support life. And we can use the closest analogues on our own
planet to guide our search.
On Earth, deep-sea vents at the boundaries between tectonic plates,
where magma breaches the sea floor, have long been recognized as
hotbeds for life. Around geysers of scalding, murky water - known as
black smokers - bacteria feed on chemicals, and all manner of
organisms make their living on those microbes.
Europa or Enceladus might just draw
enough energy from the tidal push and pull of their host planets to
have molten interiors that can fuel similar vents. We don't know.
The good news for life hunters, however, is that we're now aware of
another possibility.
When we discovered the Lost City vents beneath the Atlantic in 2000,
we saw that you can have a hydrothermal ecosystem with resident
microbes and the occasional visit from a comb jelly - the
otherworldly creature spotted by Hand during his visit - without the
faintest rumble of tectonic activity.
Lost City is powered by a chemical reaction called
serpentinisation.
The Improbable Promise of Titan
Saturn's largest moon,
Titan, boasts glorious bays and beaches,
but not a drop of liquid water.
With temperatures hovering around
-180°C, it is far too cold for that.
The lakes and seas that dot its surface are instead filled with
methane and ethane, which are gases here on Earth but slick,
oily liquids in Titan's frigid climes.
This makes Titan an unlikely focus
in the search for new forms of life in the solar system.
Astrobiologists have speculated that any life there might run on
an entirely alien chemistry. Some suggest that microbes could
make a living by breathing hydrogen and eating organic molecules
like acetylene and ethane.
The Cassini probe has spied evidence
of chemical activity in Titan's atmosphere that seems consistent
with the idea.
There could, of course, be non-biological explanations for this
activity, but the only way to know what causes it is to visit
Titan. No such mission has yet been signed off, but recent work
has given us fresh impetus by suggesting that the moon's
ice-cold chemistry would offer the toolkit required to make
weird analogues of the molecules that support life on Earth.
In 2015, a team at Cornell University in Ithaca, New York,
constructed a flexible, cell-membrane-like structure using only
the ingredients and conditions available on Titan.
Earlier this year, Martin Rahm,
also at Cornell, and colleagues did some modeling to show that
Titan should possess the chemicals required to create even more
complex molecules.
Hydrogen cyanide is abundant in Titan's atmosphere and should
rain down on the surface, but it doesn't appear to build up
there.
Instead, Rahm suggests, hydrogen
cyanide combines with other molecules when it lands, forming
larger ones made of carbon, nitrogen and hydrogen called
polyimines - and these could form the backbone of an alternative
biology.
At terrestrial temperatures, these chemical structures would
fall apart. in the cryogenic seas of Titan, however, they would
be preserved and could take on a wide array of forms, some of
which could carry out primitive versions of the reactions in
living cells here on Earth.
Rahm says they might even float to
the surface of tidal pools as membrane-like films, or as mats of
stacked, crystalline molecules. It's another leap for any such
system to be truly alive - to metabolize, replicate or evolve.
Even so, given that these chemicals can absorb light at the very
wavelengths that penetrate Titan's cloudy atmosphere, any hungry
organisms lurking in its methane seas would at least have a few
rays of sunshine to snack on.
When alkaline rocks from Earth's mantle
meet a more acidic ocean, they generate heat and spew out hydrogen,
which in turn reacts with the carbon compounds dissolved in
seawater.
It is these reactions that slowly built
the towers of carbonate, some 60 meters tall, that disgorge
organic-rich alkaline fluids into the water and make methane for
microbes to snack on.
According to Michael Russell, a geologist turned
astrobiologist
at JPL, Lost City is just the sort of place where
life on Earth might have begun.
Russell thinks that the imbalance
between the alkaline fluid flooding cell-like pores inside carbonate
chimneys and the relatively acidic seawater beyond created
electrochemical potential that the molecular precursors of life
found a way to tap. If he's right, then wherever alkaline
hydrothermal vents exist life may have followed.
Astrobiologists like Hand think there is a good chance we'll find
them on Europa and Enceladus. Now they are attempting to confirm
their suspicions from afar.
One way to do that is to look for molecules whose presence would
betray ongoing serpentinisation. Cassini's discovery of silicate
grains in Enceladus's plumes suggests this reaction has at least
happened there in the past.
Recent estimates suggesting that the
ocean itself is rather alkaline, which would be expected after eons
of serpentinisation, add to the case.
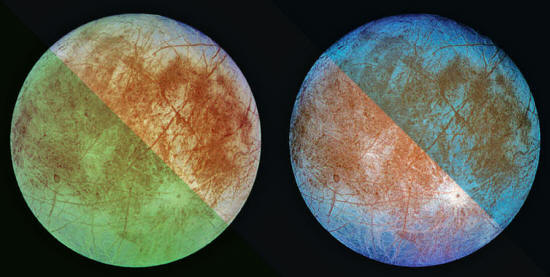
NASA/JPL/DLR
"HIDDEN OCEANS
COULD BE THE DEFAULT STATE
FOR LIFE TO ARISE,
WITH EARTH THE REAL OUTLIER"
To figure out if the process is happening today, however, we want to
see hydrogen.
That would be important because where
there are free molecules of hydrogen gas in the deep sea, there
tends to be life.
"Hydrogen is chocolate-chip cookies
for microbes," says McKay.
Although Cassini was not built to detect
molecules as large as amino acids, the probe could detect small
molecules like hydrogen.
In fact, that is precisely what it was
attempting to sniff out late last year, during its penultimate dive
through the plumes; mission scientists are still analyzing the data.
But it will be tricky to distinguish between the possible sources of
any hydrogen molecules they find.
The trouble is that hydrogen in the
plumes could
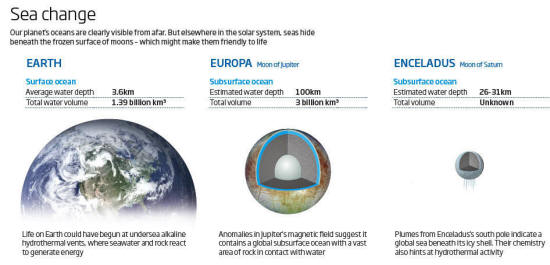
Our planet's oceans are clearly visible from afar. But elsewhere in
the solar system, seas hide beneath the frozen surface of moons -
which might make them friendly to life either be from
serpentinisation or from water split apart in the atmosphere, after
it was launched from the surface.
If it turns out to be the former, it
would be a big deal - the strongest indication yet that hydrothermal
vents at the bottom of Enceladus's ocean are serving up good amounts
of chemical fuel.
There may also be other clues in Cassini's back catalogue.
The probe flew through the plumes so
fast that it broke apart larger compounds, and we might be able to
use its detection of the fragments to reconstruct the big stuff.
"There are clearly some aromatics in
some of these heavier compounds," says Hunter Waite at the
Southwest Research Institute in San Antonio, Texas.
But aromatic compounds can be produced
through either biological or
abiotic processes, so its presence
wouldn't be a smoking gun.
Still, it would help us understand what
kinds of carbon chemistry can flourish under the ice.
Europa is even more likely to have serpentinisation as it is much
larger, meaning it boasts more rock in contact with seawater. There
are no confirmed plumes to sample, though. Instead we are learning
about its ocean chemistry by peering at its surface from telescopes
on Earth.
In October 2015, for example,
observations made with the Keck Observatory in Hawaii revealed a
strange- looking substance in a region of Europa riddled with
cracks. Although the chemical signature suggests it could be dirty
water ice, the dirty part has so far defied identification.
Patrick Fischer of the California Institute of Technology in
Pasadena, who led the analysis, says the deposits could be potassium
chloride or sodium chloride.
Both are normally transparent but could
be rendered visible by the shower of energetic particles raining
down from volcanoes on Io, Europa's explosive sister moon. If so, we
could be looking at salts left behind after underground water
breached the surface and then evaporated.
That would suggest the ocean is seasoned
not with the sulphate salts
from Io, as most people expected, but
with chloride - making it perhaps a third as salty as expected and
therefore friendlier to life.
This May, Hand and his colleagues made a bigger splash with a study
suggesting that Europa's ocean has a chemical balance similar to
Earth's. The calculations were based on estimates that fractures in
the moon's sea floor could reach as deep as 25 kilometers into the
rocky interior.
In that case, there would be great
swathes of rock surface with which water can react to release lots
of hydrogen.

Life-giving
chimneys like those at
Lost City,
under the Atlantic,
could exist on Europa
Lost City
Expedition/NSF
But that is just one part of a cycle required for life as we know
it:
electron-grabbing oxidants like
oxygen and electron-giving reducing agents like hydrogen have to
meet and react, releasing energy that living things rely on in
the form of electrons.
Europa has no atmosphere from which to
cycle oxygen, as Earth does, but we know that radiation from Jupiter
produces oxidizing chemicals on its surface.
To arrive at their conclusions about
Europa's sea, Hand and his colleagues assumed that these oxidants
are being cycled from surface to sea.
Life,
cycled
That's a big assumption.
"If you mix the subsurface and the
surface, then you get a chemical cycle that life could take
advantage of," says Britney Schmidt, an astrobiologist at the
Georgia Institute of Technology in Atlanta.
If not, life is unlikely.
And it's not yet clear whether that
cycling happens on Europa, never mind Enceladus, where the radiation
from Saturn is weaker, leaving fewer oxidants on its surface.
To find out, Britney Schmidt has drilled through Antarctic
sea ice and deployed a robotic submarine to study the underside,
where fresh ice is constantly forming and melting.
"If we can figure out how the ice
and ocean system works here on Earth, then we can extrapolate
back to Europa," she says.
Only then will we know if its vast ocean
gets enough oxidants to create the ratio of elements for life.
It is possible, of course, that life elsewhere follows a different
rulebook, that it is made from a different set of biochemical
building blocks.
So what should we be looking for if not organic
molecules and amino acids? It is a question that astrobiologists
contemplate, but it can probably only be answered by finding alien
life forms.
Maybe we never will. Maybe we really are alone in the solar system.
If we can detect something akin to deep-sea alkaline vents on
faraway moons, however, the odds of finding extraterrestrials would
be slashed.
We might also have to entertain the prospect that similarly
life-friendly conditions are lurking beneath the shells of other icy
worlds:
moons like
Ganymede,
Mimas and
Ceres.
In fact, given how common we now know
them to be, oceans concealed by frozen crusts could be the default
condition for life - in which case our blue planet, with its
peculiar open oceans, is the outlier.
|