|
from
QuantaMagazine Website the deep interdependencies of animals and plants on their microbial partners, prompting calls
for an
expansion of evolutionary theory. the fittest "individuals" when they are ecosystems made up of hosts and their microbiomes? Biologists debate the need to revise theories.
As the sky turns a deeper purple, a solitary spotted hyena awakens. She trots along the border of her clan's territory, marking the boundary with a sour paste from under her tail. She sniffs a passing breeze for hints of itinerant males interested in mating, giving little attention to her stomach's rumbling over the remnants of the previous night's hunt or the itch on her flank.
The lone hyena chooses
what she will do next to make her living.
A diverse array of
bacteria that line her gut are helping to break down her meal.
Others assist her immune system in fending off the hordes of
parasites and pathogens trying to invade her skin and other tissues.
Or does their interaction form something new, greater than each part alone?
Look closely enough at any plant or animal and you will discover a riot of bacteria, fungi and viruses forming a complex and interconnected ecosystem.
A recent explosion of
research reveals how deeply we rely on our microbial patterns to
keep our bodies functioning, raising profound questions about what
it means to be an individual.
But if our bodies are
less an autocracy of identical cells and more a coalition of
multitudes, how can we explain their evolution?
Others contend that existing theory just needs to be applied more carefully.
All agree that the micro
and macro worlds are inescapably interdependent, and that biologists
must explore the frontier of their interconnections.
Never Alone
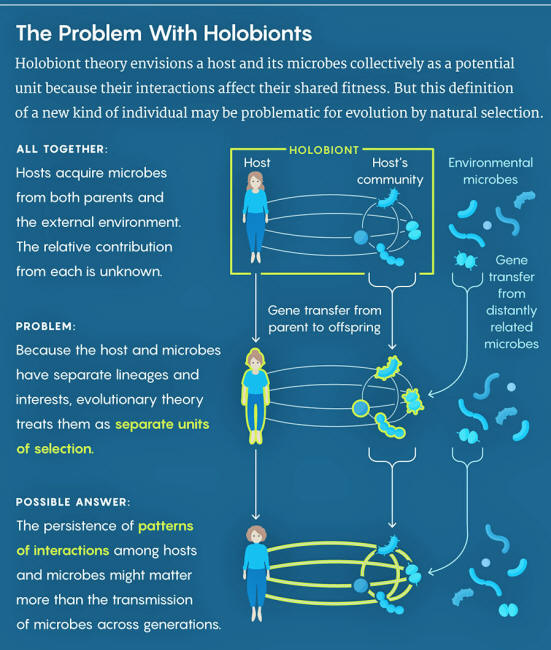
This bold assertion echoed previous calls for a reconceptualization of complex organisms as new kinds of individuals - holobionts.
The term holobiont
encompasses a host animal or plant and all its constituent microbes.
All genes within a holobiont, belonging to host or microbes,
constitute the "hologenome."
Hologenomes contain vastly more genes than the host genome alone, and since at least a fraction of the microbial genes have significant bearing on the survival and reproduction of the host, we need to consider the hologenome as a possible unit of selection if we want to understand the evolution of the holobiont.
Along with other researchers, Seth Bordenstein argues that fresh language is needed to refer to this entity, given the ubiquity of host-associated microbes in nature.
No one knows what proportion of the microbiome will influence host fitness; many are surely just along for the ride.
But if there is some degree of cooperation - for instance, if the host provides shelter or nutrients for some microbes that in return metabolize products the host can't make on its own - then they are more than just two organisms occupying the same space. They are, to a degree, functionally integrated.
And that raises the
question of whether the hologenome might also matter evolutionarily.
For such holobionts, Bordenstein says, you can't understand the evolution of either the host genome or the microbial genomes in isolation because the community of organisms as a whole shapes the traits of the individual.
The holobiont, he argues, adds up to more than the sum of the host and microbes.
Out of their
interaction emerges a coherent entity that natural selection might
act on alongside other units of selection, like the individual or a
gene.
Only when considering the holobiont as a single entity capable of being shaped as a unit by natural selection can we make sense of its complexities.
The classic recipe for evolution by natural selection begins with a population of individuals with varying characteristics that affect the number of viable offspring they are likely to have.
Those characteristics must be inheritable - that is, passed on with some fidelity from generation to generation.
A trait could
hypothetically double some lucky individual's lifespan and number of
offspring, but unless that trait gets passed on, it's an
evolutionary dead end.
Quanta
Magazine
Microbes and host genomes
can interact in ways that profoundly affect host fitness. But
whether we inherit our microbiome in something like the way we
inherit our genome remains a point of contention.
For example, females of some species of stink bug nestle a fecal pellet near freshly laid eggs to serve as the larvae's first meal, thereby inoculating them with their mother's gut microbiome.
Typically, human babies not born by cesarean section acquire their mother's vaginal microbes en route to the outside world.
Mom's microbes also rub
off on a baby through close contact and breastfeeding. Although
eventually the microbial community changes as the child moves more
freely through the world, these early microbes play an outsize role
in immune system development.
But if a significant portion of it is, he and others argue that those interactions and their evolution might be understood as a unit of selection.
Derek Skillings and other critics argue that there just isn't enough evidence of vertical transmission of symbionts to allow for the holobiont to be a coherent evolutionary individual.
Many of a host's microbes are acquired from the outside environment, not from its parents. Even when the environment is shared, there is little reason to assume that a parent's microbes will make it to its offspring.
Even if they're the same
sorts of microbes, the direct line of vertical transmission is still
necessary to form an evolutionary individual.
are there with one organism at one time
does not mean
they are a unit of selection …
Washington
University in St. Louis
Consider a host and a parasite locked in perpetual conflict: Every generation, they come together and attempt to subvert each other. You could even imagine a familial line of hosts being infected by the same familial line of parasites.
Nevertheless, persistent
as this relationship is, Skillings argues that you're only going to
understand it by considering each lineage's interests separately.
Proponents of the hologenome concept acknowledge that cooperation,
conflict and even neutrality can influence the evolution of the
holobiont, making the disagreement less about the facts of the
matter, and more about how to approach them.
Many host-associated microbes spend significant chunks of their lives outside their host, in an environment where they're subject to very different selection pressures.
The holobiont idea, she
says, puts blinders on our understanding of the evolution of these
microbes, focusing attention on the host environment and neglecting
other habitats that could shape a microbe's character.
They envision the holobiont as an ecological community, not an evolutionary individual.
The knowledge that symbiotic relationships with microbes are important,
But translating existing ecological and evolutionary theory to this new microbial world is more easily said than done, cautions Britt Koskella, a biologist at the University of California, Berkeley.
Many of these theories were built to explain how plants and animals interact and coexist, and,
Take ecological succession, a framework for evaluating how a community assembles over time.
The state of a plant
community on a new island, for example, may depend much more on the
order in which species arrived and the niches they filled than on
the local evolution of the plants, because evolution is usually so
much slower.
Bacterial succession
might work in different, counterintuitive ways from traditional
succession.
She argues that theoreticians need to think through basic assumptions made by their models and consider whether they apply equally well to microbes, and empiricists need to test the predictions of those models.
Settling empirical questions, such as how often a substantial portion of the holobiont is inherited, and how stable communities are across generations, will help sharpen intuition and inform theoretical work.
Forget about the particulars of which microbes are doing the interacting or whether they're vertically or horizontally transmitted, its proponents say - just focus on the interactions, the stable metabolic processes enacted by various microbial players.
...said in a paper (It's the Song, not the Singer - An Exploration of Holobiosis and Evolutionary Theory) W. Ford Doolittle, an evolutionary biologist at Dalhousie University in Nova Scotia.
He and his former Dalhousie colleague Austin Booth originated this idea and gave it its name, abbreviated ITSNTS, by inverting the title of a Rolling Stones song.
They argue that it
captures what's so compelling about the idea of holobionts, namely
the stable networks of interaction patterns among disparate
lineages, without ascribing a special evolutionary identity to them.
Instead, the processes themselves form a sort of evolutionary
lineage.
These networks of different players participate in metabolic cycles, in which a set of bacteria converts nutrients to metabolites, which get picked up by other bacteria to produce a different metabolite, which gets used by the host, and the cycle continues. Many of these functional steps can be carried out by myriad strains present in the gut, making any given strain potentially redundant.
But the cycle itself
continues, regardless of which cells are enacting it.
Each step in the cycle
can be carried out by innumerable species that all belong to a kind
of "functional guild," but the process itself remains remarkably
stable.
The cycle becomes a sort of structure for various lineages to grab onto, a way for them to make a living.
Doolittle and Booth liken this to the way songs perpetuate themselves as cultural entities.
Individual singers come and go, but even in cultures where written and recorded music don't exist, the songs survive by recruiting appropriate talent in new generations.
Similarly, once a
metabolic network exists, diverse lineages of organisms can evolve
to perform some of the interactions and processes that define it -
and evolution can support that association because it is selfishly
advantageous for the individuals, or genes, within the various
lineages to do so.
Cultural evolution of ideas in the form of "memes," while controversial, is seen by many as at least plausible (the meme concept was itself inspired by the biological concept of genes).
In this case, the idea or meme is the metabolic interaction, and it persists based on its ability to recruit microbes to carry it out. It remains to be seen how useful this framework might be for studying the holobiont, and significant kinks remain to be ironed out.
The notion that differential persistence is analogous to differential reproduction might seem strange to many evolutionary biologists, and it's still far from clear how to define a metabolic network.
Biologists are just starting to get a handle on the basic questions of microbe-host interactions.
Strassmann agrees.
|