|

by Team Tucker Carlson
September
17, 2021
from
TeamTuckerCarlson Website
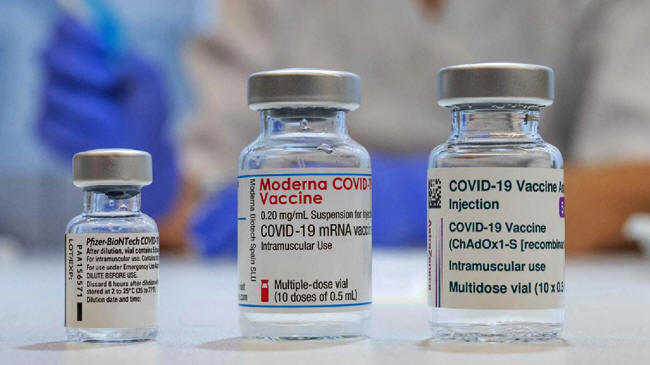
All 3
Major Vaccine Companies
suddenly have
Name Changes for their Covid Shots
...and People
are already Confused.
All three major
Covid-19 'vaccine' manufacturers
have unveiled new names for their 'fully authorized' jabs, even as
two pharmaceutical companies await FDA approval for their products.
Following the lead of
Pfizer/BioNTech, which branded its 'fully authorized' (and
oddly, unavailable) vaccine "Comirnaty," the second and third
largest manufacturers for Europe and Canada, Moderna and
AstraZeneca, are also relabeling their Covid shots.
"The Pfizer-BioNTech
vaccine is Comirnaty, the Moderna vaccine will be
named SpikeVax, and the AstraZeneca vaccine will
be named Vaxzevria," Health Canada said in a tweet
Thursday.
"These are only name
changes.
There are no changes
to the vaccines themselves," Health Canada added in an attempt
to quell confusion.
But even vaccine
supporters were confused by the name changes.
That wasn't the only thing that is raising questions, however.
Despite Pfizer having a
'fully authorized' vaccine, the vaccine manufacturer admitted that
it was still sending the Covid 'vaccine' under the label that was
initially produced under Emergency Use Authorization (EUA) in the
United States and under Interim Order Authorization in
Canada.
On Thursday, Pfizer
received 'full authorization' in Canada, as it stated in a release.
"Pfizer Canada ULC
and BioNTech SE today announced that Health Canada has granted
full approval (Notice of Compliance or NOC) for COMIRNATY® to
prevent COVID-19 in individuals 12 years of age and older,"
Pfizer said.
"The vaccine was initially authorized for use in Canada under an
Interim Order Authorization on December 9, 2020 and has
been referred to as the Pfizer-BioNTech COVID-19 Vaccine," the
release said.
"The authorization
permitted essential rollout of vaccine doses across Canada to
help provide protection during the COVID-19 'pandemic', based on
preclinical and clinical data, including initial data from the
Phase 3 clinical trial."
"Although the vaccine's brand name will be COMIRNATY following
this approval, Canada will continue to receive vials of the
vaccine labeled as Pfizer-BioNTech COVID-19 Vaccine.," Pfizer
continued.
"The formulation for
Pfizer-BioNTech COVID-19 Vaccine is the same formulation as
COMIRNATY and they are considered interchangeable by Health
Canada to provide the COVID-19 vaccination series.
Given the current
ongoing 'pandemic', a gradual transition to new labeling with
the COMIRNATY brand name will occur at a later date."
All three of the vaccine
makers' name changes have been approved for European Union
distribution.
"Spikevax it is.
Moderna has earned European Medicine Association approval for
the brand name of its COVID-19 vaccine, even as it awaits an FDA
decision," Fierce Pharma noted.
"With last week's official nod in the EU, Moderna's Spikevax
joins Pfizer and BioNTech's Comirnaty and AstraZeneca's
Vaxzevria with European brand-name approvals," the report
continued.
"None of the names is
approved in the U.S., though, because the vaccines are still
under emergency use authorization rather than bearing full FDA
approval."
"Still, it's likely the companies are fielding the same brand
names in their vaccine applications filed or soon-to-be-filed
with the FDA," the report added.
It is additionally
interesting to note how these 'vaccines' are described by the
European Medicines Agency.
"Spikevax contains a
molecule called messenger RNA (mRNA) with instructions for
producing a protein from SARS-CoV-2, the virus that causes
COVID-19," EMA said.
"Spikevax does not
contain the virus itself and cannot cause COVID-19."
"Vaxzevria is made up of another virus (of the adenovirus
family) that has been modified to contain the gene for making a
protein from SARS-CoV-2," the EMA also said.
"Vaxzevria does not
contain the virus itself and cannot cause COVID-19."
A closer examination of
the documents issued by Pfizer/BioNTech after FDA approval provides
some potential insight into why the vaccine makers want a new label
for their 'fully authorized' vaccines.
"On December 11,
2020, the Food and Drug Administration (FDA) issued an
Emergency Use Authorization (EUA) for emergency use of
Pfizer-BioNTech COVID‑19 Vaccine for the prevention of
COVID-19 for individuals 16 years of age and older pursuant to
Section 564 of the Act," the
FDA stated in a letter to the
Global Senior Director of Pfizer Ms. Elaine Harkins.
"FDA reissued the
letter of authorization on: December 23, 2020, February 25,
2021, May 10, 2021, June 25, 2021, and August 12, 2021."
"On August 23, 2021, FDA approved the biologics license
application (BLA) submitted by BioNTech Manufacturing GmbH
for COMIRNATY (COVID-19 Vaccine, mRNA) for active immunization
to prevent COVID-19 caused by SARS-CoV-2 in individuals
16 years of age and older."
PhD. biochemistry and
molecular biology student Kathleen Lee picked up on the
change in language and decided to press further.
"I called the Pfizer
BioNtech number 1-800-666-7248," she said.
"The recording
clearly states that it has not been approved by the FDA. Pfizer
BNT162B2 is still under EUA. This clears up that messy FDA
authorization between Comirnaty and Pfizer BioNtech."
"It's deceitful," Lee claimed.
"If the vial says
Comirnaty... it's FDA approved. It the vial says Pfizer
BioNtech... it's under EUA and they're not subject to
liability.
There's millions of
doses to get rid of first."
Yet if the next batch of
vaccines being dispensed were being produced under the Cominarty
label, then that would appear to reconcile the issue.
Except that isn't the case...
Buried deep within the
footnotes of the Pfizer-BioNTech documents is one footnote
that puts into rather jolting perspective that the currently labeled
vaccines are still under Emergency Use Authorization (EUA).
"Although COMIRNATY
(COVID-19 Vaccine, mRNA) is approved to prevent COVID-19
in individuals 16 years of age and older, there is not
sufficient approved vaccine available for distribution to this
population in its entirety at the time of reissuance of this EUA.
Additionally, there
are no products that are approved to prevent COVID-19 in
individuals age 12 through 15, or that are approved to provide
an additional dose to the immunocompromised population described
in this EUA."
The profound implications
of this admission was mentioned by notable vaccine critic Alex
Berenson, formerly of the New York Times and a
best-selling author on the subject.
"So now that
Come-Here-Naughty is approved (ish), the [Moderna] and [Johnson
& Johnson] lose their Emergency Use Authorizations, right?"
Berenson asked.
"Because there's an
approved alternative and you can't have an EUA when there are
'adequate, approved, and available alternatives'."
"Or is this another reason [the FDA] is going out of its way to
say Comirnaty is not available?" Berenson continued.
"[A]lthough COMIRNATY
(COVID-19 Vaccine, mRNA) is approved… there is not sufficient
approved vaccine available for distribution to this population."
Attorney Robert Barnes
also addressed the perplexing matter.
"There is no *available* FDA approved licensed vaccine," Barnes
said.
"Here's what is
happening. If FDA approved & licensed COVID19 vaccine, it would
have to revoke the EUA vaccines and subject the vaccine maker to
more liability risk.
So it only approved a
future vaccine that isn't 'available'..."
When a user questioned
his analysis, Barnes countered with an indisputable legal point.
"Did you fail to read the part of the FDA letter where it said
the licensed vaccine isn't 'available' yet?" Barnes asked.
"How is there
ANY EUA vaccines when the law does not allow them if
there is a licensed vaccine available?"
If the Pfizer-BioNTech
vaccine is a 'fully authorized' vaccine, then it should no longer be
afforded the liability protections offered by the EUA.
The other vaccines should
also cease being distributed under an EUA.
However, since this
is not the case, we can only assume that it
continues to be one giant sham...
|

