|
from QuantaMagazine Website
to support the colossal bulk of their bodies. These adaptations include special genetic mechanisms
that
make them less susceptible to cancer. until after they turned a bit of genetic junk
into a unique defense against
inevitable tumors.
A new study reveals how elephants do it:
In multicellular animals, cells go through many cycles of growth and division.
At each division, cells copy their entire genome, and inevitably a few mistakes creep in. Some of those mutations can lead to cancer. One might think that animals with larger bodies and longer lives would therefore have a greater risk of developing cancer.
But that's not what researchers see when they compare species across a wide range of body sizes:
In fact, researchers find that larger, longer-lived mammals have fewer cases of cancer.
In the 1970s, the cancer epidemiologist Richard Peto, now a professor of medical statistics and epidemiology at the University of Oxford, articulated this surprising phenomenon, which has come to be known as Peto's paradox.
The fact that larger animals like elephants do not have high rates of cancer suggests that they have evolved special cancer suppression mechanisms.
In 2015, Joshua Schiffman at the University of Utah School of Medicine and Carlo Maley at Arizona State University headed a team of researchers who showed that the elephant genome has about 20 extra duplicates of TP53, a canonical tumor suppressor gene.
They went on to suggest that these extra copies of TP53 could account, at least in part, for the elephants' enhanced cancer suppression capabilities.
Currently, Lisa M. Abegglen, a cell biologist at the Utah School of Medicine who contributed to the study, is leading a project to find out whether the copies of TP53 have different functions.
Vincent Lynch, a geneticist at the University of Chicago, has shown that part of what enabled elephants to grow so big was that one of their pseudogenes - a broken duplicate of an ancestral gene - suddenly acquired a new function. Courtesy of Vincent J. Lynch
Yet extra copies of TP53 (How Elephants Beat Cancer) are not the elephants' only source of protection.
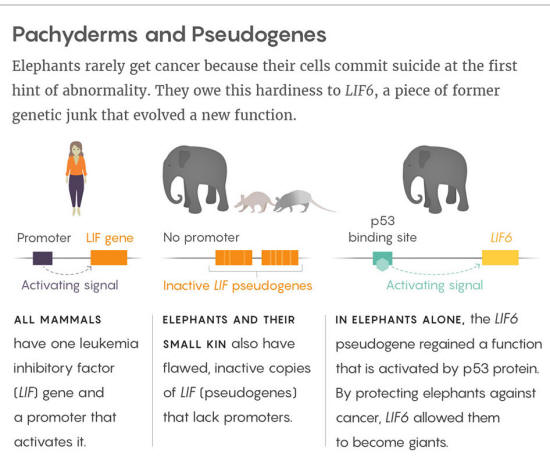
New work led by Vincent Lynch, a geneticist at the University of Chicago, shows that elephants and their smaller-bodied relatives (such as hyraxes, armadillos and aardvarks) also have duplicate copies of the LIF gene, which encodes for leukemia inhibitory factor.
This signaling protein is normally involved in fertility and reproduction and also stimulates the growth of embryonic stem cells.
Lynch presented his work (A Zombie LIF Gene in Elephants is Up-regulated by TP53 to Induce Apoptosis in Response to DNA Damage) at the Pan-American Society for Evolutionary Developmental Biology meeting in Calgary in August 2017.
Lynch found that the 11 duplicates of LIF differ from one another but are all incomplete:
These deficiencies suggested to Lynch that none of the duplicates should be able to perform the normal functions of a LIF gene, or even be expressed by cells.
But when Lynch looked in cells, he found RNA transcripts from at least one of the duplicates, LIF6, which indicated that it must have a promoter sequence somewhere to turn it on.
Indeed, a few thousand bases upstream of LIF6 in the genome, Lynch and his collaborators discovered a sequence of DNA that looked like a binding site for TP53 protein.
It suggested to them that TP53 (but not any of the TP53 duplicates) might be regulating the expression of LIF6. Subsequent experiments on elephant cells confirmed this hunch.
To discover what LIF6 was doing, the researchers blocked the gene's activity and subjected the cells to DNA-damaging conditions.
The result was that the cells became less likely to destroy themselves through a process called apoptosis (programmed cell death), which organisms often use as a kind of quality control system for eliminating defective tissue.
LIF6 therefore seems to help eradicate potentially malignant cells.
Further experiments indicated that LIF6 triggers cell death by creating leaks in the membranes around mitochondria, the vital energy-producing organelles of cells.
To find out more about the evolutionary history of LIF and its duplicates, Lynch found their counterparts in the genomes of closely related species: manatees, hyraxes and extinct mammoths and mastodons.
His analysis suggested that the LIF gene was duplicated 17 times and lost 14 times during the evolution of the elephant's lineage.
Hyraxes and manatees have LIF duplicates, but the TP53 duplicates appear only in living and extinct elephants, which suggests that the LIF duplications happened earlier in evolution.
Lynch found that most duplicates of the LIF gene are pseudo-genes - old, mutated, useless copies of genes that survive in the genome by chance.
The exception, however, is the LIF6 gene sequence, which unlike the others has not accumulated random mutations, implying that natural selection is preserving it.
That is, the elephant LIF6 re-evolved into a functional gene from a pseudo-gene ancestor.
Because it came back from the dead and plays a role in cell death, Lynch called it a "zombie gene."
Although manatees and hyraxes also have extra copies of LIF, only modern and extinct elephants have LIF6, which suggests that it evolved only after the elephants branched away from those related species.
And when Lynch's group dated the origin of LIF6 by molecular clock methods, they found that the pseudo-gene regained a function about 30 million years ago, when the fossil record indicates that elephants were evolving large body sizes.
Being able to show that it happened at roughly the same time that elephants evolved a large body, he wrote,
Evolving protections against cancer would seem to be in the interest of all animals, so why don't they all have a refunctionalized LIF6 gene?
According to the researchers, it's because this protection comes with risks.
LIF6 suppresses cancer, but extra copies of LIF6 would kill the cell if they accidentally turned on.
There also appears to be a trade-off between cancer suppression mechanisms and fertility.
A study (Single-nucleotide Polymorphisms in the p53 Pathway Regulate Fertility in Humans) published in 2009 suggested that LIF is critical for implantation of the embryo in the uterus. Because LIF activity is controlled by TP53, LIF and TP53 jointly regulate the efficiency of reproduction.
When the same set of genes has two functions (such as reproduction and cancer suppression), it is possible that those functions will be in direct conflict - a phenomenon that geneticists call antagonistic pleiotropy.
The elephants may have solved the problem of antagonistic pleiotropy by duplicating TP53 and LIF and splitting up those functions, according to Maley.
Maley speculated that the gene duplicates,
That hypothesis, however, still needs to be tested, he said.
Bats are not large animals, but some species live for decades. Scientists are investigating whether they have their own protective adaptations against cancer. Ann Froschauer, USFWS
Evolving extra copies of TP53 and LIF may have helped elephants overcome Peto's paradox, but that can't be the only solution:
Lynch and his team are currently exploring how whales and bats solve Peto's paradox.
Although not large-bodied, some bat species live up to 30 years, and the longer-lived ones might have evolved cancer suppression mechanisms that the shorter-lived ones lack.
Maley is also working on how whales solve Peto's paradox.
Even though whales don't have extra copies of TP53, he said,
Maley believes that understanding how diverse large-bodied animals solve Peto's paradox may have applications in human health.
|