|
and you'll find that it progresses much more quickly than it does over geologic time. Now the oldest viruses on the planet are enabling scientists to calibrate
this evolutionary clock.
When he compared the shapes of the bones of species separated by only a few generations, he could detect lots of small but significant changes.
Horse species separated by millions of years, however, showed far fewer differences in their morphology. Subsequent studies over the next half century found similar effects - organisms appeared to evolve more quickly when biologists tracked them over shorter timescales.
Then, in the mid-2000s, Simon Ho, an evolutionary biologist at the University of Sydney, encountered a similar phenomenon in the genomes he was analyzing.
When he calculated how quickly DNA mutations accumulated in birds and primates over just a few thousand years, Ho found the genomes chock-full of small mutations.
This indicated a briskly ticking evolutionary clock. But when he zoomed out and compared DNA sequences separated by millions of years, he found something very different.
The evolutionary clock had slowed to a crawl.
Baffled by his results, Ho set to work trying to figure out what was going on. He stumbled upon Kurtén's 1959 work and realized that the differences in rates of physical change Kurtén saw also appeared in genetic sequences.
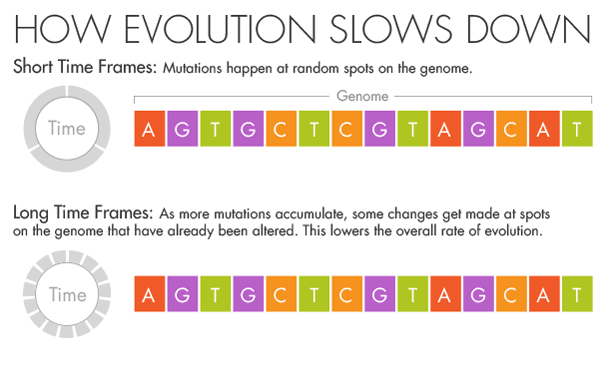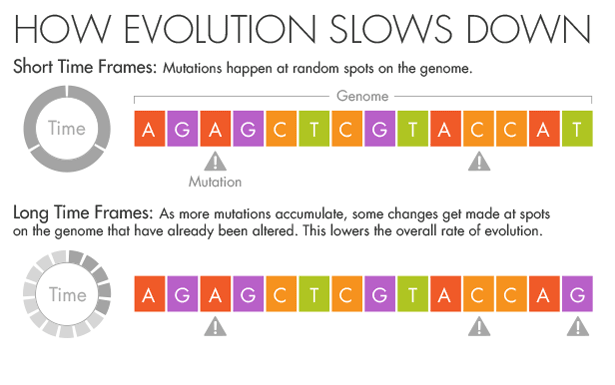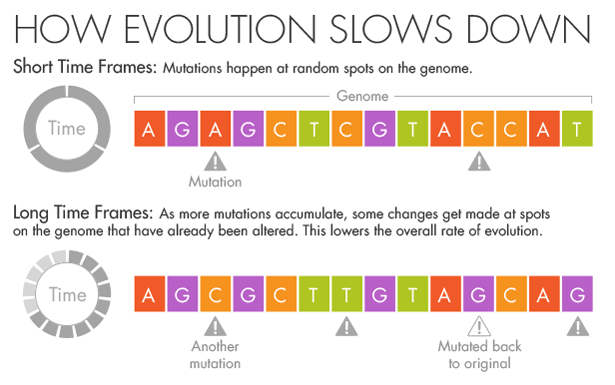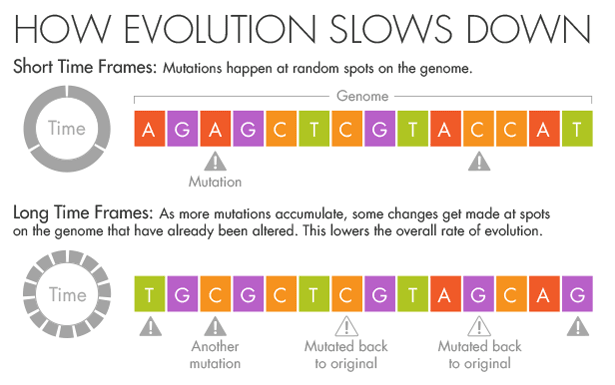
His instincts as an evolutionary biologist told him that the mutation rates he was seeing in the short term were the correct ones. The genomes varied at only a few locations, and each change was as obvious as a splash of paint on a white wall.
But if more splashes of paint appear on a wall, they will gradually conceal some of the original color beneath new layers.
Similarly, evolution and natural selection write over the initial mutations that appear over short timescales. Over millions of years, an A in the DNA may become a T, but in the intervening time it may be a C or a G for a while.
Ho believes that this mutational saturation is a major cause of what he calls the time-dependent rate phenomenon.
Simon Ho, an evolutionary biologist at the University of Sydney, found that evolution takes place at varying rates.
Courtesy of University of Sydney
Look at the hourly or daily fluctuations of Standard & Poor's 500 index, and it will appear wildly unstable, swinging this way and that.
Zoom out, however, and the market appears much more stable as the daily shifts start to average out. In the same way, the forces of natural selection weed out the less advantageous and more deleterious mutations over time.
Ho's discovery of the time-dependent rate phenomenon in the genome had major implications for biologists.
It meant that many of the dates they used as bookmarks when reading life's saga - everything from the first split between eukaryotes and prokaryotes billions of years ago to the re-emergence of the Ebola virus in 2014 - could be wrong.
The time-dependent rate phenomenon wasn't fully appreciated at first. For one thing, it is such a large and consequential concept that biologists needed time to wrap their heads around it.
But there's a bigger stumbling block:
Biologists have not been able to quantify exactly how much they should change their estimates of when things happened over the course of evolutionary history.
Without a concrete way to calculate the shifts in evolutionary rates over time, scientists couldn't compare dates.
Recently, Aris Katzourakis, a paleovirologist at the University of Oxford, has taken the time-dependent rate phenomenon and applied it to the evolution of viruses (Marine Origin of Retroviruses in the Early Palaeozoic Era).
In doing so, he has not only pushed back the origin of certain classes of retroviruses to around half a billion years ago - long before the first animals moved from the seas to terra firma - he has also developed a mathematical model that can be used to account for the time-dependent rate phenomenon, providing biologists with much more accurate dates for evolutionary events.
Other scientists are excited by the prospect.
The time-dependent rate phenomenon says that the speed of an organism's evolution will depend on the time frame over which the observer is looking at it.
And as with relativity, researchers can now calculate by how much.
Viral Fossil Hunting
Katzourakis has spent his career trying to pin down the origin of HIV and other so-called "retroviruses," which are made out of single strings of RNA.
When he looked at the mutation rates of HIV, he found that it is among the fastest-evolving viruses ever studied.
The speedy mutation rate makes sense:
Spelling errors occur on top of spelling errors.
Lucy Reading-Ikkanda Quanta Magazine
Because of this, virologists can directly study only the recent history of viruses like this.
Older samples have reached mutation saturation, with so many accumulated spelling errors that scientists can't account for them all. Taking the history of retroviruses back thousands or millions of years would require a different way to measure mutation rates.
Katzourakis turned to another technique. He searched for something akin to viral fossils inside the DNA of their hosts. Retroviruses often insert copies of their genetic material into their hosts' cells.
Most of the time, the information dies with the host.
On rare occasions, however, a retrovirus hits the evolutionary jackpot and slips inside the genome of a sperm or egg cell. Nestled securely in its host's DNA, the virus gets passed down through the generations.
Katzourakis used these viral relics to study the ancient origin of retroviruses. But when he did so, he got a big surprise.
The rate of evolution of these retroviruses over long periods appeared to slow dramatically, nearly matching that of humans and other complex life - organisms that have proofreader machinery and thus should change at a much slower pace.
If the viruses were evolving much more slowly than scientists thought, it could imply that the viruses were much older than expected as well. After all, a slowly evolving virus will need more time to change by the same amount as a quickly evolving virus.
So he set out to find an accurate date for the origin of retroviruses. To do this, he turned to a group of the most ancient retroviruses, the so-called foamy viruses, which infect everything from monkeys to cows.
This promiscuity enabled Katzourakis to calibrate his evolutionary clock to determine precisely when foamy viruses emerged.
If two species shared a foamy-virus sequence, the virus must have infected their common ancestor, before the two species diverged.
Aris Katzourakis, a paleovirologist at the University of Oxford, dated a class of viruses to the era before the sea-to-land transition.
Gillman & Soame
Researchers in labs around the world had slowly pushed back the date of origin of foamy viruses to 100 million years ago.
But Katzourakis found hints that the virus had infected reptiles, amphibians and even fish far earlier than 100 million years ago. To conclusively show that retroviruses were older than the accepted date of 100 million years, however, Katzourakis would need to date the virus itself.
He dived into Ho's papers on the time-dependent rate phenomenon, hoping to figure out how to apply it to viruses. He also wanted to create a general model that would allow researchers to input the timescale they were observing and get back details about the organism's evolutionary rate.
Katzourakis and his student Pakorn Aiewsakun tried out four different ways to quantify how quickly the evolutionary rate appeared to change based on timescale.
They found that a power law rate-decay model fit their data best and showed that evolutionary rates decrease exponentially as the timescale increases. A subsequent study of 396 different viruses revealed that the evolutionary rate slows at the same rate across almost all genome types and replication strategies.
Existing evolutionary clocks, which fail to account for the time-dependent rate phenomenon, inaccurately date ancient viruses as being much younger than they really are.
Katzourakis and Aiewsakun then used the newly developed mathematical framework to recalculate the emergence of foamy viruses.
Using their newly developed model, the scientists showed in a paper (Marine origin of retroviruses in the early Palaeozoic Era) published in January that foamy viruses emerged somewhere between 460 and 550 million years ago. Independent work by the University of Arizona virologist Michael Worobey, published (Foamy-like endogenous retroviruses are extensive and abundant in teleosts) in Virus Evolution nearly simultaneously, also suggested that these viruses originated earlier than expected.
These studies established the oldest date for any known group of viruses, although Katzourakis believes other viral groups may be even more ancient.
The findings have implications far beyond the earning of a trophy for the oldest virus.
A convergence on the same date of origin for foamy viruses provides evidence that the time-dependent rate phenomenon isn't just a relic of statistics or the methods researchers use to date species.
Katzourakis's model also gives researchers a tool to quantify the effects of the time-dependent rate phenomenon, which will prove key to understanding the factors that drive this phenomenon.
More broadly, the work by Katzourakis and Ho challenges the idea of a steadily ticking evolutionary clock.
It also means that scientists may need to revise the dates of evolutionary events in the deep past, as they likely underestimated how long ago they truly happened, Katzourakis said.
He is trying to understand whether the pruning of mutations by natural selection and mutational saturation is the sole contributor to the time-dependent rate phenomenon, or whether other factors play a role in how and why the phenomenon emerges.
|