|
04 April 2011
An electrochemically responsive membrane
promises improved treatments for chronic diseases.
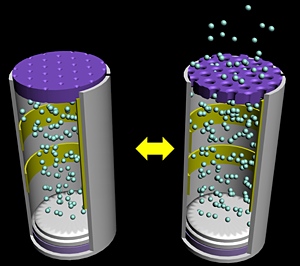
Schematic illustration of an electrically activated drug delivery device. In the reduced state (left), the pores are closed due to the expansion of the membrane.
In the oxidized state
(right), the pores open to release the device’s drug payload.
Pulsatile drug delivery devices can provide controlled and long-term release of drugs in the body under the action of an external stimulus, offering the promise of new treatments for chronic diseases that require daily injections or precise doses of toxic medication.
In a new development for such biomedical
devices, Jin Kon Kim from the Pohang University of Science and
Technology in Korea and colleagues have designed a porous membrane
that stores and releases drug molecules according to an external
electric signal.
According to Kim, the counterions that bind to the negatively charged membrane play an important role in the opening/closing of the pores by maintaining the neutrality of the PPy chains at each state.
Moreover, the team found that, unlike for other electrically responsive materials, they could very quickly and reversibly switch from one state to another more than 1,000 times.
They also managed to load the membrane
with large amounts of a model protein drug.
|