|

by Kelly Kennedy
March 05, 2012
from
USATODAY Website

Pfizer spent
$12 million lobbying Congress in 2011
WASHINGTON
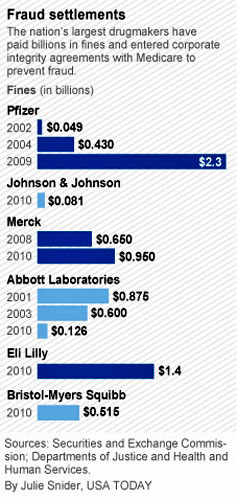
The nation's largest drugmakers have
paid at least $8 billion in fines for repeatedly defrauding Medicare
and Medicaid over the past decade, but they remain in business with
the federal government because they are often the sole suppliers of
critical products, records show.
Pfizer, the maker of drugs that help
alleviate arthritis and other ailments, has paid almost $3 billion
in fines since 2002 and entered into three corporate integrity
agreements with the Department of Health and Human Services
aimed at preventing future fraud.
It and other companies are fighting
attempts by Congress to exclude them from government business
because of their history of fraud.
Merck, another pharmaceutical giant, paid $1.6 billion in fines
since 2008, Medicare and Justice Department records show, to resolve
claims it was not paying proper rebates to the government.
Pfizer's 2009 settlement was for improperly promoting the use of
drugs for purposes other than those for which they were approved by
the government. Merck's 2008 settlement involved claims the company
paid illegal kickbacks to health care providers in exchange for
prescribing its drugs.
Government investigators say their hands are tied with the tools
they have. They can exclude Pfizer and other pharmaceutical
companies from providing medications to Medicaid and Medicare
beneficiaries as punishment for bad behavior, but that would leave
beneficiaries without drugs patented through a particular company.
Or they can fine the companies and force them to enter corporate
integrity agreements that require government oversight and a promise
not to defraud the government again - a promise that often goes
unkept.
"We're seeing some of the big
companies a second and third time," said Gregory Demske,
assistant inspector general for legal affairs for Health and
Human Services.
"The corporate integrity agreement
is not sufficient to deter further misconduct."
In addition, the cases are labor- and
cost-intensive as the companies fight often for years to avoid an
exclusion, Demske said.
To try to change that trend, the government announced in 2010 that,
rather than exclude an entire company, investigators would go after
individuals within a company.
Demske said,
his organization, the Justice
Department and the Food and Drug Administration have come up
with some ideas to use within the scope of the rules - such as
taking away a company's patent rights as a condition of a
settlement.
That could begin with cases being
investigated now, he said.
Sen. Chuck Grassley, R-Iowa,
introduced a bipartisan bill that would make it easier for the
government to find a middle ground, saying the law now forces,
"the inspector general to use
all-or-nothing, mandatory exclusion penalties against
corporations that have committed fraud."
The bill would allow the exclusion of
individuals from working with the government even after they've left
the company where the fraud occurred.
Pharmaceutical companies altogether spent more than $200 million
lobbying Congress in 2011, including $12 million spent by Pfizer. At
least 12 pharmaceutical and medical device companies are lobbying
specifically against a House bill, HR 675, that complements
Grassley's.
None of the pharmaceutical companies,
-
Abbott Laboratories
-
Pfizer
-
Bristol-Myers Squibb,
... contacted by USA TODAY responded to
questions about their response to the government's proposed
enforcement actions.
The industry's trade group, the Pharmaceutical Research and
Manufacturers of America, says excluding an individual should
occur only when there is "significant wrongdoing" that the
individual knew about and did nothing to stop, said Matthew
Bennett, the group's senior vice president.
|


