Antibiotic Overuse
- Stop the Killing of
Beneficial Bacteria -
by Martin Blaser
Nature
24 August 2011
Source
Spanish version

Concerns about antibiotics focus on bacterial resistance - but
permanent changes to our protective flora could have more
serious consequences, says Martin Blaser.
The average child in the United States and other developed
countries has received 10-20 courses of antibiotics by the time
he or she is 18 years old.1 In many respects,
this is a life-saving development.
The average US citizen born in 1940
was expected to live to the age of 63; a baby born today should
reach 78, in part because of antibiotics. But the assumption
that antibiotics are generally safe has fostered overuse and led
to an increase in bacterial resistance to treatments.
Other, equally serious, long-term consequences of our love of
antibiotics have received far less attention. Antibiotics kill
the bacteria we do want, as well as those we don't.
Early evidence from my lab and
others hints that, sometimes, our friendly flora never fully
recover. These long-term changes to the beneficial bacteria
within people's bodies may even increase our susceptibility to
infections and disease.
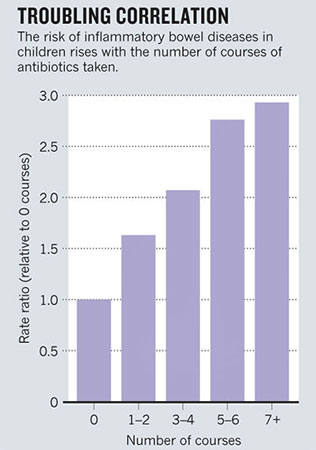
Overuse of antibiotics could be
fuelling the dramatic increase in conditions such as obesity,
type 1 diabetes, inflammatory bowel disease, allergies and
asthma, which have more than doubled in many populations (see
graph).
We urgently need to investigate this possibility.
And, even before we understand the
full scope, there is action we should take.
Bacteria have lived in and on animals - constituting their
microbiome - since multicellular life evolved about 1 billion
years ago. Hosts derive many benefits from their bacterial
guests:2
the Bacteroides species that
dwell in the colon synthesize our required vitamin K; gut
bacteria help us to resist invading organisms.
An oral or injectable antibiotic
diffuses through the bloodstream and affects targeted pathogen
and residential microbiota alike.
And evidence is accumulating that
our welcome residents do not, in fact, recover completely3 or
are replaced in the long term by resistant organisms.4
Collateral damage
In the early twentieth century, Helicobacter pylori was the
dominant microbe in the stomachs of almost all people.
By the turn of the twenty-first
century, fewer than 6% of children in the United States, Sweden
and Germany were carrying the organism. Other factors may be at
play in this disappearance5, but antibiotics may be a culprit.
For example, a single course of
amoxicillin or a macrolide antibiotic, most commonly used to
treat middle-ear or respiratory infections in children, may also
eradicate
H. pylori in 20-50% of cases.
"Each generation could be
beginning life with a smaller endowment of ancient microbes
than the last."
In humans, eradicating H. pylori
affects the regulation of two hormones produced in the stomach
and involved in energy balance, ghrelin and leptin.
And as H. pylori has disappeared
from people's stomachs, there has been an increase in
gastroesophageal reflux, and its attendant problems such as
Barrett's oesophagus and oesophageal cancer.
Could the trends be linked?
H. pylori is a risk factor for peptic ulcers and stomach cancer,
but a microbe probably wouldn't have been so pervasive if it
didn't carry some benefit to its host.
Indeed, large studies we performed
have found that people without the bacterium are more likely to
develop asthma, hay fever or skin allergies in childhood.6
Stomachs that lack H. pylori seem
immunologically quite different from those that do not, and
infection of young mice with H. pylori protects against
experimental asthma.7
There is other evidence that antibiotics cause shifts in
microbial composition that may bring long-term physiological
changes.
For instance, as farmers have
discovered, continuous, sub-therapeutic doses of many different
antibacterial agents cause animals to gain weight with less
food.
And the earlier that antibiotics are
started, the more profound the effects. In my laboratory, we
have preliminary evidence in a mouse model that changes in body
fat and tissue composition are associated both with low-dose
antibiotic treatment that mimics farm use, and with high-dose
treatment similar to those used to treat childhood infections.
The changes in our microbiome may even be fuelling the
transmission of deadly organisms such as methicillin-resistant
Staphylococcus aureus 5 and Clostridium
difficile.8
This is not an enormous surprise,
because one of the important roles of an intact microbial
ecosystem is to resist intrusions by pathogenic organisms.
To better understand the long-term effects of antibiotic use, we
need to compare the microbiomes of antibiotic-using and
antibiotic-free populations.
We are working with Maria Gloria
Dominguez Bello at the University of Puerto Rico in San Juan
and her colleagues to study people living in remote regions in
the Amazon who either have never received antibiotics or who
have had very limited recent exposures.
If antibiotics do cause long-term physiological changes, we may
not be able to wait until we fully understand the problem before
changing our approaches.
Knowledge gleaned from farms
indicates that early life is most crucial, triggering
physiological changes that are difficult to reverse later on.
Consequently, we should reduce the use of antibiotics during
pregnancy and childhood. Antibiotics - particularly penicillins
- are now given routinely to between one-third and one-half of
all women during pregnancy or nearing childbirth in the United
States and other developed countries.
Babies acquire their founding
bacterial populations from their mothers while passing through
the vagina at birth.
So each generation - particularly
the 30% or so of infants born via Caesarian 9
- could be beginning life with a smaller endowment of ancient
microbes than the last.5
When antibiotics seem warranted - such as in the 30% of pregnant
women with group B Streptococcus, which causes serious infection
in about 1 in 200 newborns - we must better assess which mothers
need to be treated, or whether a vaccine might be preferable.
Targeted attack
Another precautionary step would be to develop specific agents
to stabilize at-risk residential microbial populations, such as
effective
probiotics.
We also need new, narrow-spectrum
antibacterial agents to minimize collateral effects on the
microbiota. This is an admittedly huge task, which will require
providing incentives for the pharmaceutical industry to develop
targeted classes of antibacterial agents and, importantly,
better diagnostics that rapidly identify the problematic agent.
We may also need to start replacing what has been lost over the
past 70 years.
Along with receiving standard
vaccinations, for instance, one day, children whose microbiome
has been genotyped could be given inoculations of specific
strains of H. pylori to reduce their chance of later developing
allergies or asthma, then receive narrow-spectrum antibiotics
later in life to eliminate the bacterium and lower the risks of
peptic ulceration and gastric cancer.
The ease of worldwide travel is increasing our global
vulnerability to pathogens, just as our ancient microbial
defences are eroding.
We must make use of the available
technology to protect and study our bacterial benefactors before
it is too late.
References
Source
-
Sharland, M. J. Antimicrob.
Chemoth. 60 (suppl. 1), i15–i26 (2007).
-
Ley, R. E., Lozupone, C. A.,
Hamady, M., Knight, R. & Gordon, J. I. Nature Rev.
Microbiol. 6, 776–788 (2008).
-
Dethlefsen, L. & Relman, D.
A. Proc. Natl Acad. Sci. USA 108 (suppl. 1), 4554–4561
(2011).
-
Sjölund, M., Wreiber, K.,
Andersson, D. I., Blaser, M. J. & Engstrand, L. Ann.
Intern. Med. 139, 483–487 (2003).
-
Blaser, M. J. & Falkow, S.
Nature Rev. Microbiol. 7, 887–894 (2009).
-
Chen. Y. & Blaser, M. J.
Arch. Intern. Med. 167, 821–827 (2007).
-
Arnold, I. C. et al. J. Clin.
Invest. 121, 3088–3093 (2011).
-
Chang, J. Y. et al. J.
Infect. Dis. 197, 435–438 (2008).
-
Dominguez-Bello, M. G. et
al. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).