|

by James Gallagher
Health and science
reporter
28 August 2013
from
BBCNews Website
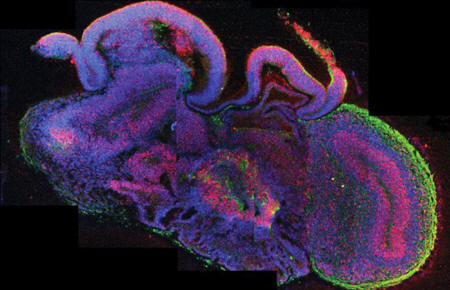
Cross-section
of miniature human brains
termed cerebral
organoids
Miniature "human brains" have been grown in a lab in a feat
scientists hope will transform the understanding of neurological
disorders.
The pea-sized structures reached the same level of development as in
a nine-week-old fetus, but are incapable of thought.
The study,
published in the journal Nature,
has already been used to gain insight into rare diseases.
Neuroscientists have described the findings as astounding and
fascinating. The human brain is one of the most complicated
structures in the universe.
Scientists at Institute of Molecular Biotechnology of the
Austrian Academy of Sciences have now reproduced some of the
earliest stages of the organ's development in the laboratory.
Brain bath
They used either embryonic stem cells or adult skin cells to produce
the part of an embryo that develops into the brain and spinal cord -
the neuroectoderm.
This was placed in tiny droplets of gel to give a scaffold for the
tissue to grow and was placed into a spinning bioreactor, a nutrient
bath that supplies nutrients and oxygen.
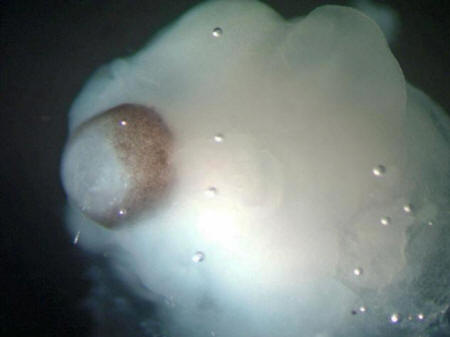
A cerebral organoid
The brown pigments
are a developing retina
The cells were able to grow and organize themselves into separate
regions of the brain, such as the cerebral cortex, the retina, and,
rarely, an early hippocampus, which would be heavily involved in
memory in a fully developed adult brain.
The researchers are confident that this closely, but far from
perfectly, matches brain development in a fetus until the nine week
stage.
The tissues reached their maximum size, about 4mm (0.1in), after two
months.
The "mini-brains" have survived for nearly a year, but did not grow
any larger. There is no blood supply, just brain tissue, so
nutrients and oxygen cannot penetrate into the middle of the
brain-like structure.
One of the researchers, Dr Juergen Knoblich, said:
"What our organoids are good for is
to model development of the brain and to study anything that
causes a defect in development.
"Ultimately we would like to move towards more common disorders
like schizophrenia or autism. They typically manifest themselves
only in adults, but it has been shown that the underlying
defects occur during the development of the brain."
The technique could also be used to
replace mice and rats in drug research as new treatments could be
tested on actual brain tissue.
'Mindboggling'
Researchers have been able to produce brain cells in the laboratory
before, but this is the closest any group has come to building a
human brain.
The breakthrough has excited the field.
Prof Paul Matthews, from Imperial College London, told the
BBC:
"I think it's just mindboggling. The
idea that we can take a cell from a skin and turn it into, even
though it's only the size of a pea, is starting to look like a
brain and starting to show some of the behaviors of a tiny
brain, I think is just extraordinary.
"Now it's not thinking, it's not communicating between the areas
in the way our brains do, but it gives us a real start and this
is going to be the kind of tool that helps us understand many of
the major developmental brain disorders."
The team has already used the
breakthrough to investigate a disease called
microcephaly.
People with the disease develop much
smaller brains.
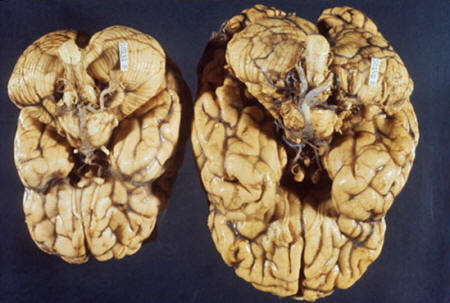
Brain with
microcephaly
A much smaller brain
develops with microcephaly
By creating a "mini-brain" from skin cells of a patient with this
condition, the team were able to study how development changed.
"It's a long way from conscience or awareness
or responding to the
outside world.
There's always the spectre of what
the future might hold,
but this is
primitive territory"
Dr Zameel Cader
John Radcliffe Hospital
They showed that the cells were too keen
to become neurons by specializing too early. It meant the cells in
the early brain did not bulk up to a high enough number before
specializing, which affected the final size of even the pea-sized
"mini-brains".
The team in Vienna do not believe there are any ethical issues at
this stage, but Dr Knoblich said he did not want to see much larger
brains being developed as that would be "undesirable".
Dr Zameel Cader, a consultant neurologist at the John
Radcliffe Hospital in Oxford, said he did not see ethical issues
arising from the research so far.
He told the BBC:
"It's a long way from conscience or
awareness or responding to the outside world. There's always the
spectre of what the future might hold, but this is primitive
territory."
The "mini
brain"
is roughly the
size and developmental level of a nine-week fetus
Dr Martin Coath, from the
cognition institute at Plymouth University, said:
"Any technique that gives us
'something like a brain' that we can modify, work on, and watch
as it develops, just has to be exciting.
"If the authors are right - that
their 'brain in a bottle' develops in ways that mimic human
brain development - then the potential for studying
developmental diseases is clear. But the applicability to other
types of disease is not so clear - but it has potential.
"Testing drugs is, also, much more problematic. Most drugs that
affect the brain act on things like mood, perception, control of
your body, pain, and a whole bunch of other things. This
brain-like-tissue has no trouble with any of these things yet."
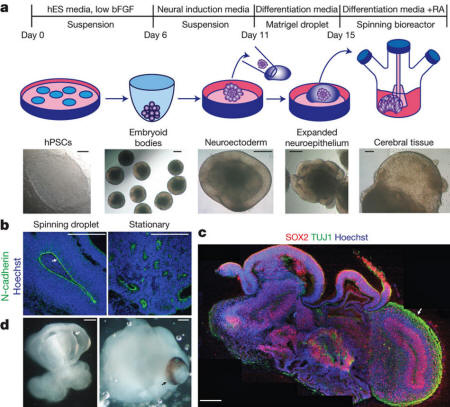
Description of cerebral
organoid culture system
a - Schematic of
the culture system described in detail in Methods.
Example images of each stage are shown. bFGF, basic
fibroblast growth factor; hES, human embryonic stem
cell; hPSCs, human pluripotent stem cells; RA,
retinoic acid.
b - Neuroepithelial tissues generated using this
approach (left) exhibited large fluid-filled
cavities and typical apical localization of the
neural N-cadherin (arrow). These tissues were larger
and more continuous than tissues grown in stationary
suspension without Matrigel (right).
c - Sectioning
and immunohistochemistry revealed complex morphology
with heterogeneous regions containing neural
progenitors (SOX2, red) and neurons (TUJ1, green)
(arrow).
d - Low-magnification bright-field images revealing
fluid-filled cavities reminiscent of ventricles
(white arrow) and retina tissue, as indicated by
retinal pigmented epithelium (black arrow). Scale
bars, 200 μm.
|




