|
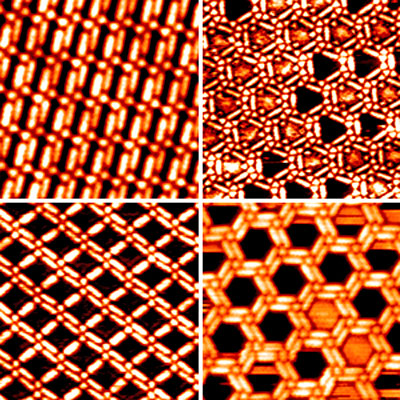
Scanning tunneling microscopy images
showing various TMA–BPBP networks Non-covalent bonds formed by electrostatic interactions rather than the sharing of electrons define the structure and function of proteins and other large biological molecules.
Such bonds, which include hydrogen bonds
and ionic bonds, have also become pivotal in the development of
materials at the nanoscale. Kai Wu and colleagues from Peking
University and the Zhejiang University of Technology in China have
now created two-dimensional molecular porous networks with a
systematically adjustable structure using the hierarchical formation
of hydrogen bonds.1
Wu and his colleagues took an alternative approach by using a single set of building blocks - trimesic acid and a multi-ringed bipyridine-like molecule called BPBP - to create a range of architectures simply by adjusting the ratio of block types.
Mimicking non-covalently bound systems found in nature, the researchers exploited hydrogen bonds of various strengths to modify the network geometry.
The team first deposited trimesic acid on a gold surface to form a chicken-wire structure, then vaporized an equal concentration of BPBP over the top.
By altering the relative proportion of the molecular components on the surface and the substrate temperature, they were able to produce a variety of networks (see image), including linear, triangular, diamond and hexagonal pore shapes.
He adds that the cavities can serve as micro-reactors for quantitative studies of surface reactions. The team is also investigating ways to insert spin-carrying metallic atoms or ions into the pores to control the spin state of the assemblies.
Author affiliation
|