|
October 24, 2012
With the wicked flurry of news about the Food and Drug Administrationís Monster Energy Drink investigation, thereís been a lot of follow-on news about the amount of caffeine in energy drinks.
But as a comment in a recent piece about energy drinks pointed out, thereís much more in these beverages than just caffeine and water.
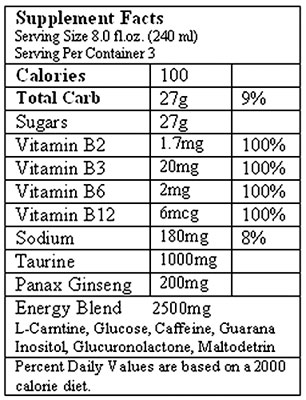
So, I thought it would be interesting to dissect the ingredient labels of typical energy drinks and see what else weíre ingesting with every swig. Below is the ingredient label for an 8 ounce can of Monster Energy:
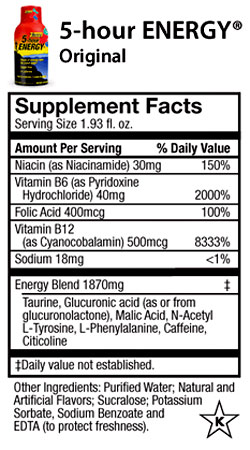
And below is the ingredient label for a 5-Hour Energy shot:
Iím using these two examples because they cover most of the common ingredients found in energy drinks and shots.
The first thing worth noticing is that both contain a handful of B vitamins, which may or may not have energy boosting effects depending on which research source you consult, followed by an ďEnergy BlendĒ that contains multiple ingredients, none of which are broken out individually by milligrams. Monsterís blend has a total of 2500 mg; 5-Hour Energyís has 1870 mg.
Letís tour some of the ingredients in these blends and see what weíre drinking.
Overall, these ingredients fall in the not dangerous and probably not useful category, with a couple of exceptions.
Worth noting, caffeine is definitely not the only stimulant in either of these drinks - or others on the market - so itís probable that the FDA investigation may lead to further ingredient probing.
Monster Energy Drink
from
Reuters Website
The Food and Drug Administration (FDA) said on Monday that it was investigating reports of five deaths that may be associated with Monster Beverage Corp's namesake energy drink, and the company's shares fell more than 14 percent.
Monster is also being sued by the family of a 14-year-old Maryland girl with a heart condition who died after drinking two cans of its Monster energy drink in a 24-hour period.
Monster said it does not believe its drinks are "in any way responsible" for the girl's death.
The energy drink market is dominated by Monster and Austrian company Red Bull, but it also includes beverages made by Coca-Cola Co and PepsiCo.
The lawsuit and reports of other deaths that may be associated with energy drinks illustrate safety concerns surrounding the highly caffeinated beverages that are especially popular with young people.
They could also embolden the industry's critics, including two senators and the New York attorney general.
That could mean more extensive labeling requirements or age restrictions, Mullarkey said.
He added that the negative headlines also made Monster a less attractive takeover target.
Sources told Reuters in April that the two companies had discussed a possible deal as recently as last year.
CONCERNS NOT NEW
The family of Anais Fournier sued Monster on Friday for failing to warn about the product's dangers.
The lawsuit, filed in California Superior Court in Riverside, said that after drinking two 24-ounce cans of Monster Energy on consecutive days Fournier went into cardiac arrest. She was placed in an induced coma and died six days later on December 23, 2011.
The lawsuit said Fournier died from "cardiac arrhythmia due to caffeine toxicity" that complicated an existing heart valve condition related to a disorder called Ehlers-Danlos syndrome.
The two drinks together contained 480 milligrams of caffeine, the equivalent of 14 12-ounce cans of Coca-Cola, according to the lawsuit.
On Monday, FDA spokeswoman Shelly Burgess said the agency had received reports of five deaths and one heart attack that may be associated with the Monster energy drink from 2009 through June this year.
The FDA said it investigates any report of injury or death that it receives. The notices to the FDA's adverse events database do not in themselves confirm a risk from a product.
Burgess said manufacturers are required to submit all reports on serious adverse events to the FDA within 15 days of receiving them, and that they are responsible for providing follow-up information that could shed light on their cause.
Last month, Senators Dick Durbin of Illinois and Richard Blumenthal of Connecticut sent a letter to the FDA asking it to investigate the interaction of ingredients in energy drinks and the effect of the caffeine on children and adolescents. The letter followed a similar request from Durbin in April.
In July, New York Attorney General Eric Schneiderman issued subpoenas to three energy drink makers,
... seeking information on the companies' marketing and advertising practices.
PepsiCo makes the AMP energy drink, and Living Essentials makes 5-Hour Energy.
The combination of caffeine and alcohol came into the spotlight two years ago when a handful of college students were hospitalized for alcohol poisoning after drinking alcoholic energy drinks like Four Loko. Four Loko's maker later removed the caffeine from the drinks.
Monster is the leading U.S. energy drink by volume with nearly 39 percent of the U.S. market, but Austria's Red Bull has the highest share by revenue due to its premium price.
Monster Energy drinks are sold in the United States and Europe, and the company is rolling them out to,
It said in August that it was planning more international launches next year. The company had net sales of $592.6 million in the second quarter, ended on June 30.
Monster shares closed down 14.23 percent at $45.73 on the Nasdaq.
The case is Crossland et al v. Monster Beverage Corp, California Superior Court, Riverside County, No. RIC1215551.
|