|

by Peter Doshi
16 May 2013
from
TheRefusers Website
MB Comment
by The Refusers
May 17, 2013
Here is a new hard-hitting
article from the British Medical Journal (BMJ) that
claims the CDC is lying about flu vaccine effectiveness
and destroying its own credibility.
The Refusers obviously agree
with this conclusion. The public needs to wake up and
reject the media onslaught urging annual flu
vaccination.
It’s based on junk science,
commercial interests and outright corruption among
so-called scientific authorities who care more about
feathering their own nests than facts.
Julie Gerberding
Former CDC head, current
chief Merck vaccine huckster

Julie Gerberding
Former CDC
head,
current chief
Merck vaccine huckster
The best example of this sordid
situation is Julie Gerberding, the head of Merck
vaccines (the largest US vaccine manufacturer) - who is the
former head of the CDC.
The CDC mission is
to push vaccines,
no matter how useless and dangerous they may be.
The best career hope for CDC
flunkies is to collaborate with that corrupt program and
make a jump to a better-paid position with a drug company, a
path blazed by vaccine huckster Gerberding.
The entire BMJ article is below, after this brief Q & A with
the author.
Q&A with study author Peter Doshi,
Harvard University
Q) Should we continue to get
the flu shot? What about parents who are trying to
decide for their children?
A) Public health experts are routinely misleading the
public as to the strength of the science in support of
its statements about vaccine effectiveness, safety, and
the threat of influenza… The vaccine is not always
"better than nothing."
Q) Could you comment on the studies that show that
getting the flu shot may not prevent you from getting
sick, but can help prevent you from getting a serious
case?
A) My paper addresses the studies that claim influenza
vaccines reduce the risk of influenza complications. No
good studies support this claim… I would encourage you
to take specific questions to the CDC regarding its
policy.
The CDC pledges,
“To base all public health decisions
on the highest quality scientific data, openly and objectively
derived.”
But Peter Doshi argues that in
the case of influenza vaccinations and their marketing, this is not
so. Promotion of influenza vaccines is one of the most visible and
aggressive public health policies today.
Twenty years ago, in 1990, 32 million
doses of influenza vaccine were available in the United States.
Today around 135 million doses of influenza vaccine annually enter
the US market, with vaccinations administered in drug stores,
supermarkets - even some drive-throughs.
This enormous growth has not been fueled
by popular demand but instead by a public health campaign that
delivers a straightforward,
who-in-their-right-mind-could-possibly-disagree message: influenza
is a serious disease, we are all at risk of complications from
influenza, the flu shot is virtually risk free, and vaccination
saves lives.
Through this lens, the lack of influenza
vaccine availability for all 315 million US citizens seems to border
on the unethical.
Yet across the country, mandatory
influenza vaccination policies have cropped up, particularly in
healthcare facilities,1 precisely because not everyone
wants the vaccination, and compulsion appears the only way to
achieve high vaccination rates.2
Closer examination of influenza vaccine
policies shows that although proponents employ the rhetoric of
science, the studies underlying the policy are often of low quality,
and do not substantiate officials’ claims.
The vaccine might be less beneficial and
less safe than has been claimed, and the threat of influenza appears
overstated.
Now we are all
“at risk” of serious complications
Influenza vaccine production has grown parallel to increases in the
perceived need for the vaccine. In the US, the first recommendations
for annual influenza vaccination were made in 1960 (Table1).
Through the 1990s, the key objective of
this policy was to reduce excess mortality. Because most of
influenza deaths occurred in the older population, vaccines were
directed at this age group. But since 2000, the concept of who is
“at risk” has rapidly expanded, incrementally encompassing greater
swathes of the general population (Box 1 below).
As one US Centers for Disease Control
and Prevention (CDC) poster picturing a young couple warns:
“Even healthy people can get the
flu, and it can be serious.” 3
Today, national guidelines call for
everyone 6 months of age and older to get vaccinated. Now we are all
“at risk.”
View this table:
Table 1.
Expansion of influenza
vaccination recommendations, 1960 to present
|
Population |
1960 |
1984 |
1987 |
2000 |
2004 |
2006 |
2008 |
2009 |
2010 |
|
Recommendations
by age |
|
|
|
|
|
|
|
|
|
|
Adults ≥ 65
years |
X |
X |
X |
X |
X |
X |
X |
X |
X |
|
Adults ≥ 50
years |
|
|
|
X |
X |
X |
X |
X |
X |
|
Children 6 to 23
months |
|
|
|
|
X |
X |
X |
X |
X |
|
Children 6 to 59
months |
|
|
|
|
|
X |
X |
X |
X |
|
Children 6
months to 18 years, if feasible |
|
|
|
|
|
|
X |
X |
X |
|
Children 6
months to 18 years |
|
|
|
|
|
|
|
X |
X |
|
Everyone ≥ 6
months |
|
|
|
|
|
|
|
|
X |
|
Recommendations
by condition or occupation |
|
|
|
|
|
|
|
|
|
|
Pregnant women
(2nd and 3rd trimester) |
|
|
|
X |
X |
X |
X |
X |
X |
|
Pregnant women
(all trimesters) |
|
|
|
|
X |
X |
X |
X |
X |
|
Healthcare
workers |
|
X |
X |
X |
X |
X |
X |
X |
X |
|
Household
contacts of high risk groups |
|
|
X |
X |
X |
X |
X |
X |
X |
|
Household
contacts and out of home
caregivers of children 0-23 months |
|
|
|
|
X |
X |
X |
X |
X |
|
Household
contacts and out of home
caregivers of children 0-59 months |
|
|
|
|
|
X |
X |
X |
X |
|
Box 1 - A policy without an objective
Despite the enormous sums of money spent fighting the perceived
threat of influenza, there are surprisingly few instances of
unambiguous statements describing the objectives of influenza
vaccination policy.
Here is a sampling, drawn from more than
five decades of influenza vaccination policies in the United States,
that demonstrates the changing purpose of the campaign - from one
with a clear objective of saving older people’s lives, to one
without any stated objective.
In 1964, four years after annual influenza vaccination policies were
first instituted, CDC influenza branch chief Alexander Langmuir and
colleagues wrote that the recommendation,
“was based on three broad
assumptions:
-
That excess mortality was the most important
consequence of epidemic influenza.
-
That polyvalent virus vaccines
had been at least partially effective in preventing clinical illness
during most epidemics and therefore presumably would reduce the risk
of death among the aged and chronically ill.
-
That epidemics
cannot be predicted with sufficient accuracy to permit confident
planning of control measures on a year to year basis.” 4
In 1984,
recommendations from the Advisory Committee on Immunization
Practices stated:
“Because of the increasing proportion of elderly
persons in the United States and because age and its associated
chronic diseases are risk factors for severe influenza illness, the
future toll from influenza may increase, unless control measures are
used more vigorously than in the past...
For about 20 years,
efforts to reduce the impact of influenza in the United States have
been aimed primarily at immunoprophylaxis [vaccination] of persons
at greatest risk of serious illness or death.” 5
Today, the
recommendations do not even mention the effect the policy aims to
achieve.6
Box 2 - Deciphering the numbers
As concern surged this January over a worse than usual influenza
season, members of the media seemed unsure whether the CDC’s
announcement that “vaccine effectiveness (VE) was 62%” 7 represented
good versus disappointing news.8
NBC anchor Brian Williams:
“I worry about this number. I woke up to
reports of this number. It can disincentivize people to go get that
flu shot which all of you are saying is still so important.”
Chief medical editor Nancy Snyderman:
“And I had the same concern
when you see 62%, because I’m afraid people will say ‘well, it’s
half and half.’
But remember, if you have a 62% less chance of
getting of getting the flu, it means less chance of being on
antibiotics, less chance of ending up in an intensive care unit, and
as we’ve seen from this uptick in numbers, 62% less chance of
dying.” 9
Although the study never tested more severe outcomes such as
hospitalizations and death, the logic is nonetheless tempting: if
62% fewer people get influenza, then would not one expect 62% fewer
of all of influenza’s complications? Not necessarily so.
The reason
is that the 62% reduction statistic almost certainly does not hold
true for all subpopulations. In fact, there are good reasons to
assume it does not.
It is well known that influenza infections are
more severe for certain groups of people, such as the frail older
population, compared with others like healthy young adults.
The CDC
study did not present the statistics by age or health status, but an
update of the study released one month later showed 90% of
participants were younger than 65 years, and for older people, there
was no significant benefit (vaccine effectiveness was 27%; 95%
confidence interval, 31% to 59%).10
Not to worry - officials say influenza vaccines save lives
Risk of serious illness is a problem - but, according to the
official narrative, a tractable problem, thanks to vaccines.
As
another CDC poster, this time aimed at seniors, explains:
“Shots
aren’t just for kids. Vaccines for adults can prevent serious
diseases and even death.”11
And in its more technical guidance
document, CDC musters the evidence to support its case. The agency
points to two retrospective, observational studies.
One, a 1995
peer-reviewed meta-analysis published in Annals of Internal
Medicine, concluded:
“many studies confirm that influenza vaccine
reduces the risks for pneumonia, hospitalization, and death in
elderly persons during an influenza epidemic if the vaccine strain
is identical or similar to the epidemic strain.”12
They calculated a
reduction of “27% to 30% for preventing deaths from all causes” -
that is, a 30% lower risk of dying from any cause, not just from
influenza.
CDC also cites a more recent study published in the New
England Journal of Medicine, funded by the National Vaccine Program
Office and the CDC, which found an even larger relative reduction in
risk of death: 48%.13
If true, these statistics indicate that influenza vaccines can save
more lives than any other single licensed medicine on the planet.
Perhaps there is a reason CDC does not shout this from the rooftop:
it’s too good to be true. Since at least 2005, non-CDC researchers
have pointed out the seeming impossibility that influenza vaccines
could be preventing 50% of all deaths from all causes when influenza
is estimated to only cause around 5% of all wintertime deaths.14 15
So how could these studies - both published in high impact, peer
reviewed journals and carried out by academic and government
researchers with non-commercial funding - get it wrong?
Consider one
study the CDC does not cite, which found influenza vaccination
associated with a 51% reduced odds of death in patients hospitalized
with pneumonia (28 of 352 [8%] vaccinated subjects died versus 53
deaths among 352 [15%] unvaccinated control subjects).16
Although
the results are similar to those of the studies CDC does cite, an
unusual aspect of this study was that it focused on patients outside
of the influenza season - when it is hard to imagine the vaccine
could bring any benefit.
And the authors, academics from Alberta,
Canada, knew this: the purpose of the study was to demonstrate that
the fantastic benefit they expected to and did find - and that
others have found, such as the two studies that CDC cites - is
simply implausible, and likely the product of the “healthy-user
effect” (in this case, a propensity for healthier people to be more
likely to get vaccinated than less healthy people).
Others have gone
on to demonstrate this bias to be present in other influenza vaccine
studies.17 18 Healthy user bias threatens to render the
observational studies, on which officials’ scientific case rests,
not credible.
Yet for most people, and possibly most doctors, officials need only
claim that vaccines save lives, and it is assumed there must be
solid research behind it.
But for those that bother to read the
CDC’s national guidelines19 - a 68 page document of 33 360 words and
552 references - one finds that the evidence cited is these
observational studies that the agency itself acknowledges may be
undermined by bias.
The guidelines state:
“...studies demonstrating large reductions in hospitalizations
and deaths among the vaccinated elderly have been conducted using
medical record databases and have not measured reductions in
laboratory-confirmed influenza illness.
These studies have been
challenged because of concerns that they have not controlled
adequately for differences in the propensity for healthier persons
to be more likely than less healthy persons to receive
vaccination.”19
CDC does not rebut or in any other way respond to these criticisms.
It simply acknowledges them, and leaves it at that.
If the observational studies cannot be trusted, what evidence is
there that influenza vaccines reduce deaths of older people - the
reason the policy was originally created? Virtually none.
Theoretically, a randomized trial might shine some light - or even
settle the matter.
But there has only been one randomized trial of
influenza vaccines in older people - conducted two decades ago - and
it showed no mortality benefit (the trial was not powered to detect
decreases in mortality or any complications of influenza).
This
means that influenza vaccines are approved for use in older people
despite any clinical trials demonstrating a reduction in serious
outcomes. Approval is instead tied to a demonstrated ability of the
vaccine to induce antibody production, without any evidence that
those antibodies translate into reductions in illness.
Perhaps most perplexing is officials’ lack of interest in the
absence of good quality evidence.
Anthony Fauci, director of the US
National Institute of Allergy and Infectious Diseases, told the
Atlantic that,
it “would be unethical” to do a placebo controlled
study of influenza vaccine in older people.20
The reason? Placebo
recipients would be deprived of influenza vaccines - that is, the
standard of care, thanks to CDC guidelines.
This is not to say influenza vaccines have no proven benefit. Many
randomized controlled trials of influenza vaccines have been
conducted in the healthy adult population, and a systematic review
found that, depending on vaccine-virus strain match, vaccinating
between 33 and 100 people resulted in one less case of influenza.21
No evidence exists, however, to show that this reduction in risk of
symptomatic influenza for a specific population - here, among
healthy adults - extrapolates into any reduced risk of serious
complications from influenza such as hospitalizations or death in
another population (complications largely occur among the frail,
older population).
This fact seems hard for many health commentators
to grasp, who seem all too ready to take the largest statistic and
apply it to all outcomes for all populations.
At a press briefing
this winter, CDC director Thomas Frieden said a preliminary CDC
study had found,
“the overall vaccine effectiveness to be 62%.”
He
explained that this estimate of relative risk reduction:
“means that
if you got vaccinated you’re about 60% less likely to get the flu
that requires you to go to your doctor.”
On the evening news, the
CDC’s message was translated into a claim that influenza vaccines
will cut the risk of death by 62%, despite the fact that the CDC
study did not even measure mortality (Box 2,
far above).
Reflecting on the same
CDC study, two authors editorialized in the Journal of the American
Medical Association that there exists an irrational pessimism about
influenza vaccine:
“A prevention measure that reduced the risk of a
serious outcome by 60% in most instances would be a noted
achievement; yet for influenza vaccine, it is seen as a ‘failure.’”
Here, too, the authors appear unaware that the CDC study they cite
did not measure any “serious outcome” like pneumonia, only medically
attended acute respiratory illness with influenza confirmed by the
laboratory.
Officials say
influenza vaccines are safe
The CDC’s universal influenza vaccination recommendation carries the
implicit message that, beyond those for whom the vaccine is
contraindicated, influenza vaccine can only do good; there is no
need to weigh risks against benefits.
In October 2009, the US
National Institutes of Health produced a promotional YouTube video
featuring Fauci.
Urging US citizens to get vaccinated against
the
H1N1 influenza, Fauci stressed the vaccine’s safety:
“the track
record for serious adverse events is very good. It’s very, very,
very rare that you ever see anything that’s associated with the
vaccine that’s a serious event.”
Months later, Australia suspended its influenza vaccination program
in under five year olds after many (one in every 110 vaccinated)
children had febrile convulsions after vaccination.
Another serious
reaction to influenza vaccines - and also unexpected - occurred in
Sweden and Finland, where H1N1 influenza vaccines were associated
with a spike in cases of narcolepsy among adolescents (about one in
every 55 000 vaccinated).
Subsequent investigations by governmental
and non-governmental researchers confirmed the vaccine’s role in
these serious events.22 23 24 25
Selling sickness - what’s in a name?
Drug companies have long known that to sell some products, you would
have to first sell people on the disease.
Early 20th century
advertising for the mouthwash Listerine, for example, warned readers
of the problem of “halitosis” - thereby turning bad breath into a
widespread social concern.26
Similarly, in the 1950s and 1960s,
Merck launched an extensive campaign to lower the diagnostic
threshold for hypertension, and in doing so enlarging the market for
its diuretic drug,
Diuril (chlorothiazide).27
Today drug companies
suggest that we have under-diagnosed epidemics of erectile
dysfunction, social anxiety disorder, and female sexual dysfunction,
each with their own convenient acronym and an approved medication at
the ready.
Could influenza - a disease known for centuries, well
defined in terms of its etiology, diagnosis, and prognosis - be yet
one more case of disease mongering? I think it is.
But unlike most
stories of selling sickness, here the salesmen are public health
officials, worried little about which brand of vaccine you get so
long as they can convince you to take influenza seriously.
Marketing influenza vaccines thus involves marketing influenza as a
threat of great proportions.
The CDC’s website explains that,
“Flu
seasons are unpredictable and can be severe,” citing a death toll of
“3000 to a high of about 49 000 people.”
However, a far less
volatile and more reassuring picture of influenza seems likely if
one considers that recorded deaths from influenza declined sharply
over the middle of the 20th century, at least in the United States,
all before the great expansion of vaccination campaigns in the
2000s, and despite three so-called “pandemics” (1957, 1968, 2009)
(fig 1).
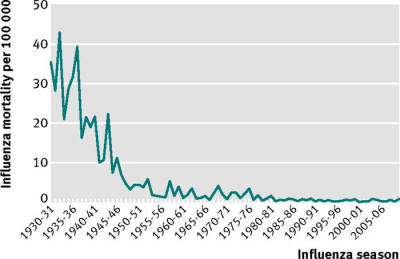
Fig 1
Crude mortality per
100 000 population, by influenza season
(July to June of the
following year),
for seasons 1930-31
to 2009-10, US.
Data sources: Doshi
P. Am J Pub Health 2008;98:939-45.
But perhaps the cleverest aspect of the influenza marketing strategy
surrounds the claim that “flu” and “influenza” are the same.
The
distinction seems subtle, and purely semantic.
But general lack of
awareness of the difference might be the primary reason few people
realize that even the ideal influenza vaccine, matched perfectly to
circulating strains of wild influenza and capable of stopping all
influenza viruses, can only deal with a small part of the “flu”
problem because most “flu” appears to have nothing to do with
influenza.
Every year, hundreds of thousands of respiratory
specimens are tested across the US. Of those tested, on average 16%
are found to be influenza positive. (fig 2).
All influenza is “flu,” but only one in six “flues” might be
influenza.
It’s no wonder so many people feel that “flu shots” don’t
work: for most flues, they can’t.
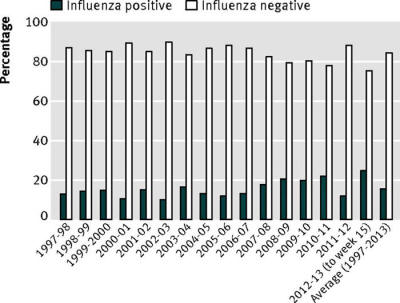
Fig 2
Proportion of
specimens testing positive for influenza at
World Health
Organization (WHO)
Collaborating
Laboratories and National Respiratory
and Enteric Virus
Surveillance System (NREVSS) laboratories
through the United
States.
Data are compiled and
published by CDC.28-43
Notes
Footnotes
-
Acknowledgements: I am grateful
to Yuko Hara, Tom Jefferson, and Edward Davies, for their
comments.
-
Competing interests: I have read
and understood the BMJ Group policy on declaration of
interests and declare the following interests: PD is a
co-recipient of a UK National Institute for Health Research
grant to carry out a Cochrane review of neuraminidase
inhibitors (http://www.hta.ac.uk/2352). PD received €1500
from the European Respiratory Society in support of his
travel to the society’s September 2012 annual congress where
he gave an invited talk on oseltamivir. He is funded by an
institutional training grant from the Agency for Healthcare
Research and Quality (AHRQ) #T32HS019488. AHRQ had no role
in study design, data collection and analysis, decision to
publish, or preparation of the manuscript.
-
Provenance and peer review:
commissioned: not externally peer reviewed.
References
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
|



