|
Nothing captures the imagination like the idea of a memory implant.
Not some impossible chip that somehow resurrects vanished memories, but rather a little something extra that gives the brain an edge when it comes to laying down new memories.
All this assumes of course that brains actually store things as opposed to merely creating answers to queries on the fly. As the latest effort to build some kind of "memory bridge" now takes form at Lawrence Livermore National Labs (LLNL), we might ask - can such a device actually work?
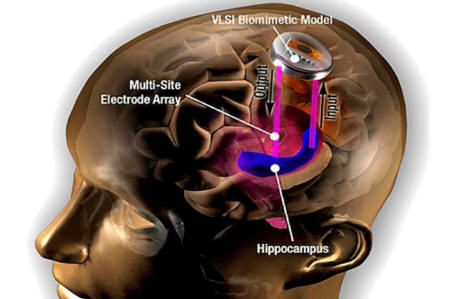
Until now the only serious effort to build an implantable memory device was that of Ted Berger's lab at USC.
As we have seen, with just a limited set of electrodes in hippocampal regions and no real clue as to what kinds of codes are involved (hint: the code is there are no codes), they have their work cut out for them.
The LLNL group, which previously contributed to the groundbreaking Argus II retinal prosthesis is now taking a more integrated approach.
With the recent announcement of ample federal BRAIN Initiative funding, they aim to build multifunction electro-optical-chemical neural sensor-effectors.
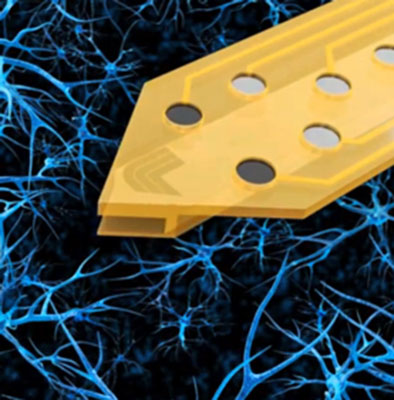
On the electrical end, LLNL's new wafer technology will use fairly high electrode counts, perhaps 500-1000 spots.
While higher density 11,000-electrode chips have been par for the course in single neuron analysis, these chips will have more sparsely distributed electrode locations.
Integrated light guides will provide conduits for optogenetic manipulations, and as an added bonus bi-directional fluid channels for any number of chemical exchanges are also etched in.
To wire everything up, new standardized multiplexed connectors are also in the works.
LLNL engineer Vanessa Tolosa holds up a brain implant
The end application is veterans with traumatic brain injury - over a quarter-million of them. That is a pretty large potential test pool.
To show they are serious, the DARPA funders have brought in a real-world implant heavyweight Medtronic to lend its expertise and devices.
Medtronic has been touting the capabilities of its closed-loop Aptiva PC+S stimulators which in theory will use locally recorded neural data to control the stimulation. The problem is that for now there is no clearly defined feedback algorithm that has been publicly floated, if in fact there is even one in private.
As far as any outsider can tell this ideally "instantaneous" control loop, if it is ever going to be closed, is going to route through Medtronic headquarters itself before coming back to the brain.
So for now, it's still go, go, go on the hardware end, but little progress it seems on intuitive grasp of the stimulation pipeline that would have anything to do with memory.
In other words, where do you put the thing exactly, and what neurons precisely do you might want to control? We might suppose that in the greater scheme of all things neural, brain cells might be born equal, but they do not end up equal.
Control hierarchies and preferred activation paths will inevitably emerge. In the same vein any implant that is willy-nilly plopped into the brain should either identify and target these kingpin cells, or create them itself.
This might, for example, by done by dispensing various chemical incentives and affirmants through that integrated port we mentioned. What these treats and rewards might actually be remains to be seen.
One other technology we want to mention here, one that will really make precisely-targeted deep brain stimulation (DBS) possible, is to have a real 3D model of the regions of the brain involved.
One of the most promising efforts in this regard is some new work by Michele Tagliati's group from the Movement Disorders Program in the department of neurology at Cedars-Sinai.
They have created 3D anatomical models of several patient brains from MRI scans to aid in the placement of DBS electrodes. Using multiphysics simulations they were then able input actual stimulation parameters to optimize the effects.
While this type of thing has been done for decades for building better cochlear implants, only now is it beginning to be applied to the brain at large in a clinical setting.
|