|
The Lancet Volume 354, Issue 9179, Page 684 21 August 1999 from TheLancet Website
...Expressing
Galanthus Nivalis Lectin on Rat Small Intestine The Lancet Volume 354, Issue 9187, Pages 1353 - 1354 16 October 1999 from TheLancet Website
Diets containing genetically modified (GM) potatoes expressing the lectin Galanthus nivalis agglutinin (GNA) had variable effects on different parts of the rat gastrointestinal tract.
Some effects, such as the proliferation of the gastric mucosa, were mainly due to the expression of the GNA transgene. However, other parts of the construct or the genetic transformation (or both) could also have contributed to the overall biological effects of the GNA-GM potatoes, particularly on the small intestine and caecum.
GM potatoes expressing a snowdrop lectin (Galanthus nivalis agglutinin [GNA]) under the CaMV35s promoter have been developed to increase insect and nematode resistance.1
GNA was selected for insertion into potatoes because the initial effect of this mannose-specific lectin on the rat small bowel has been shown to be minimal,2 and because its binding to mannose present on the epithelial surface of rat jejunal villi is demonstrable only after feeding for 10 days.
We compared the histological indices of the gut of rats fed potato diets containing GM potatoes, non-GM potatoes, or non-GM potatoes supplemented with GNA, to find out whether GNA gene insertion had affected the nutritional and physiological impact of potatoes on the mammalian gut.
Six rats were randomly allocated to each group, and were fed diets containing either raw or boiled GNA-GM potatoes, parent potatoes (Desiree), or parent-line potatoes supplemented with 25.4 μg/g GNA for 10 days. All potato diets were isocaloric and contained an average of 6% protein.
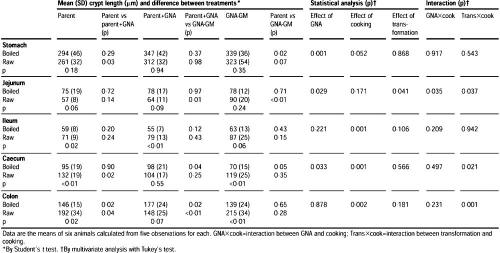
Histological samples of stomach, jejunum, ileum, caecum, and colon were taken 10 days after the start of feeding.
The samples, each 2 cm in length, were opened along the antimesenteric border. The serosal surface was allowed to adhere to card for 3 min and was then fixed in 10% neutral buffered formalin for 18 h at 20°C. Paraffin sections (4 μm) were stained with haematoxylin and eosin, and mucosal thickness (stomach) or crypt length (jejunum, ileum, caecum, and colon) was measured by video-image analysis.
Intraepithelial lymphocytes are equally distributed in all parts of the small intestine, and are known to increase when non-specific intestinal damage occurs. Thus, to assess potential damage, intraepithelial lymphocytes were counted in eight jejunal villi from each of the six rats fed diets containing GNA-GM potatoes or parent potatoes, both raw and boiled.
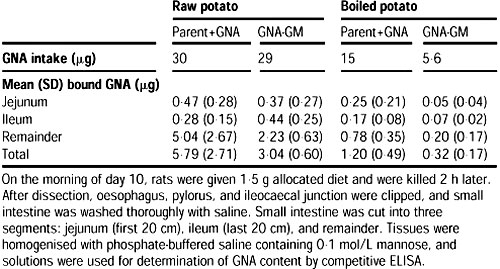
No such measurements were made for the group fed parent potatoes spiked with GNA because dietary GNA or other lectins do not induce lymphocyte infiltration. GNA binding to the jejunum and ileum was measured by elution with 0.1 mol/L mannose, followed by ELISA.
This effect was observed with both raw and boiled potatoes. Crypt length in the jejunum of rats fed on raw GNA-GM potato diets was significantly greater than in those given parent-line or parent-line plus GNA potato diets. However, the increase in jejunal crypt length was not seen in rats fed boiled GNA-GM potatoes (table 1).
GNA had no significant effects on the ileum, but rats fed boiled potatoes had shorter ileal crypts than rats given respective raw potato diets. Rats fed boiled GNA-GM potatoes had significantly thinner caecal mucosae than rats given boiled parent potatoes, with or without GNA supplementation (table 1).
Intraepithelial lymphocyte counts per 48 villi were 7.6 (SD 2.7) in rats fed on boiled parent potatoes, compared with 10.3 (3.3) in rats fed boiled transgenic potatoes (p<0.01). With raw potato diets, the intraepithelial lymphocyte counts were again significantly different: 5.3 (2.0) and 9.3 (2.6) in parent and GM potatoes, respectively (p<0.01).
Peyer's patches appeared normal in all rats. GNA binding in the jejunum and ileum was about the same, irrespective of whether spiked GNA potatoes or GM potatoes were fed (table 2).
Measurement of GNA binding by immunocytochemistry also showed a similar pattern.2 Effect of raw and cooked parent, parent+GNA, and GNA+GM potatoes on histological indices of rat gut
Table 2 GNA binding to the jejunum and ileum of rats given diets containing GNA-GM potatoes or parent potato diets spiked with GNA
Thus, we propose that the unexpected proliferative effect was caused by either the expression of other genes of the construct, or by some form of positioning effect in the potato genome caused by GNA gene insertion.
Because caecal thickness was similar in rats given boiled parent potatoes in the presence or absence of spiked GNA, we suggest that the decrease in caecal mucosal thickness seen in rats fed boiled GM-potato diets was the consequence of the transfer of the GNA gene into the potato. Caecal mucosal thickness in rats given raw potato diets was significantly higher than in those given the corresponding boiled potatoes. Thus, the main effect of boiling was to decrease mucosal thickness; this binding was fully in line with expectations.
The raw parent-line potato diets supplemented with GNA were associated with a significantly thinner caecal mucosa than that of rats given parent-line potato diets.
A similar trend was also observed in rats fed raw GNA-GM potatoes, but the difference did not reach significance (table 1).
Rats fed on GNA-supplemented parent potatoes had significantly shorter colonic crypt lengths than those fed on parent potatoes of GNA-GM potatoes; the reason for this finding is not clear.
Other parts of the GM construct, or the transformation, could have contributed to the overall effects.
Once bound, GNA is internalized by endocytosis,2 some other component of the construct in the GNA-GM potato or its expressed gene product might also be able to penetrate and affect the rat mucosal cells in a similar manner. The growth-promoting effect of raw GNA-GM potatoes in the jejunum, evident as crypt hyperplasia, is probably due to a direct stimulatory effect on crypt cells; the increase in T lymphocyte infiltration may be important in the elimination of damaged enterocytes.3
The possibility that a plant vector in
common use in some GM plants can affect the mucosa of the
gastrointestinal tract and exert powerful biological effects may
also apply to GM plants containing similar constructs, particularly
those containing lectins, such as soya beans or any plants
expressing lectin genes or transgenes.
This study was supported by Scottish
Office: Agriculture, Environment, and Fishery Department (grant
number FF 818).
References
Feeding GMO Potatoes To Rats
from
YouTube Website
Dr. Arpad Pusztai discusses his research on the effects of feeding GMO potatoes to rats.
Additional Information
|