|
|
|
13 December 2010 from NatureAsia Website
An aqueous lithium-ion battery can be
recharged a thousand times with marginal capacity loss.
In contrast, the lithium-ion batteries typically found in laptops and mobile phones can pack a large amount of energy into a small space, and continue to work well after thousands of recharging cycles.
Lithium-ion batteries in which lithium ions are transported between the positive and negative electrodes through an aqueous solution could be used to power electric vehicles.
This recharge capacity comes at a cost,
however, because the flammable organic solvent used in lithium-ion
batteries to carry electrical current between electrodes makes the
batteries relatively expensive and somewhat explosive - factors that
have prevented larger lithium-ion batteries from being used in
vehicular applications.
This technology could enable much larger lithium-ion batteries to be used safely in electric vehicles.
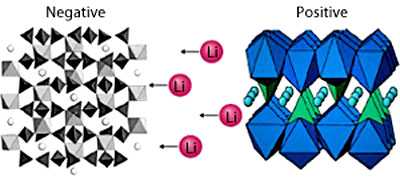
As the battery charges, lithium ions are
added to the negative electrode, which is made from lithium titanium
phosphate. On discharging, lithium ions leave this electrode and
travel through the electrolyte - in this case an aqueous solution of
lithium sulfate - before inserting into the positive electrode,
which is made from carbon-coated lithium iron phosphate.
Operating at 1.4 V, the battery could be
completely charged and discharged a thousand times with only 10%
loss in capacity.
The
scientists are now developing ways to prevent the generation of
oxygen in the battery when it is overcharged by limiting the maximum
voltage of the positive electrode.
Reference
|