|

by Anne Marie Helmenstine, Ph.D.
May 2008
from
ChemistryAbout Website
|
It's possible to
remove fluoride
from drinking water,
but not every type of
water filter will work.
|
Most people are aware
that there is a controversy surrounding public fluoridation of
drinking water. Here is a list of ways to obtain drinking water
without fluoride.
In addition, I've listed
water purification methods which do not remove fluoride from water.
Ways to Remove
Fluoride from Water
-
Reverse Osmosis Filtration
- This is used to purify several types of bottled water (not
all), so some bottled waters are unfluoridated. Reverse
osmosis systems are generally unaffordable for personal use.
-
Activated
Alumina Defluoridation Filter - These filters are used in
locales where fluorosis is prevalent. They are relatively
expensive (lowest price I saw was $30/filter) and require
frequent replacement, but do offer an option for home water
filtration.
-
Distillation
Filtration - There are commercially available distillation
filters that can be purchased to remove fluoride from water.
On a related note:
When looking at bottled
water, keep in mind that 'distilled water' does not imply that a
product is suitable for drinking water and other undesirable
impurities may be present.
These Do NOT
Remove Fluoride
-
Brita, Pur, and
most other filters - Some websites about fluoride removal
state otherwise, but I checked the product descriptions on
the companies' websites to confirm that fluoride is left in
the water.
-
Boiling Water -
This will concentrate the fluoride rather than reduce it
-
Freezing Water -
Freezing water does not affect the concentration of fluoride
Steps to
Reduce Fluoride Exposure
-
Don't take
fluoride supplements
-
Read labels on
bottled beverages - Unless they are made using distilled or
reverse-osmosis water, they are probably made with
fluoridated public water
-
Consider using
unfluoridated toothpaste
-
Avoid drinking
black or red tea - There are many health benefits associated
with chemical compounds found in tea, but this may be a
beverage to avoid if you need to reduce your fluorine
intake. Black and red tea come from two different types of
plants, but both leaves naturally contain high amounts of
fluorine
-
Be wary of
tinned fish and canned food items - Fluoride may be used as
a preservative
-
Avoid black or
red rock salt or items containing black or red rock salt
-
Avoid using
chewing tobacco
-
Void
long term use of medication that contains fluorine - Certain
antidepressants and medications for osteoporosis contain
fluorine
from
ProjectJhabua Website
The defluoridation methods are divided into three basic types
depending upon the mode of action :
-
Based on some
kind of chemical reaction with fluoride: Nalgonda technique,
Lime...
-
Based on
adsorption process: Bone charcoal, processed bone,
tricalcium phosphate, activated carbons, activated magnesia,
tamarind gel, serpentine, activated alumina, plant
materials, burnt clay...
-
Based on
ion-exchange process: Anion/Cation exchange resins
Filtration:
-
Reverse Osmosis
Filtration
-
Activated
Alumina Defluoridation Filter
-
Distillation Filtration
-
|
Method
|
Process
|
Resources / Salient
Features
|
|
Nalgonda
Technique |
The
Nalogonda technique (named after the village in India
where the method was pioneered) employs flocculation
principle 1. Nalgonda technique is a combination of
several unit operations and the process invloves rapid
mixing, chemical interaction, floculation,
sedimentation, filtration, disinfection and sludge
concentration to recover waters and aluminium salts.
Alum (hydrated aluminium salts) - a coagulant commonly
used for water treatment is used to flocculate fluoride
ions in the water. Since the process is best carried out
under alkaline conditions, lime is added. For the
disinfection purpose bleaching powder is added. After
thorough stirring, the chemical elements coagulate into
flocs and settle down in the bottom. The reaction occurs
through the following equations
2 Al2 (SO4)3 . 18H2 O + NaF + 9Na2CO3 →
[5Al(OH)3.Al(OH)2F] + 9Na2SO4+NaHCO3 + 8 CO2 + 45 H2O 3
Al2 (SO4)3 . 18H2 O + NaF +17NaHCO3 →
[5Al(OH)3.Al(OH)2F] + 9Na2SO4+ 17 CO2 + 18 H2O
|
Salient
features of Nalgonda technique
-
No
regeneration of media
-
No
handling of caustic acids and alkalis
-
Readily
available chemicals used in conventional municipal
water treatment are only required
-
Adaptable to domestic use
-
Flexible
up to several thousands m3 / d
-
Applicable in batch as well as in continuous
operation to suit needs simplicity of design,
construction, operation and maintenance
-
Local
skills could be readily employed
-
Higly
efficient removal of fluorides from 1.5 to 20 mg/L
to desirable levels
-
Simultaneous removal of color, odor, turbidity,
bacteria and organic contaminants
-
Normally
associated alkalinity ensures fluoride removal
efficiency
-
Sludge
generated is convertible to alum for use elsewhere
-
Little
wastage of water and least disposal problem
-
Needs
minimum of mechanical and electrical equipment
-
No
energy except muscle power for domestic equipment
-
Economical - annual cost of defluoridation (1991
basis) of water at 40 lpcd works out to Rs.20/- for
domestic treatment and Rs.85/- for community
treatment using fill and draw system based on 5000
population for water with 5 mg/L and 400 mg/L
alkalinity which requires 600 mg/L alum dose.
-
Provides
defluoridated water of uniform acceptable quality
|
|
Precipitation methods |
Method
involving the addition in sequence, of an alkali,
chlorine and aluminium sulphate or aluminium chloride or
both was developed. It is cheap and is used extensively
in India.
Though lime
softening accomplishes fluoride removal, its high
initial cost, large dosage and alkaline pH of the
treated water renders it unsuitable for field
application. Large dosage and alkaline pH of the treated
water renders it unsuitable for field application.
|
Alkali,
chlorine;
Aluminium
sulphate or aluminium chloride |
|
Activated
alumina |
Activated
alumina is a granular, highly porous material consisting
essentially of aluminum trihydrate. It is widely used as
a commercial desiccant and in many gas drying processes.
The studies,
perhaps the earliest, have demonstrated the high
potential of activated alumina for fluoride uptake. An
initial concentration of 5 mg/L was effectively brought
down to 1.4 mg/L before regeneration and to 0.5 mg/L on
regeneration with 2N HCl. The bed was regenerated with a
solution of 2% Na OH,5% NaCl,2N HCl,5% NaCl and 2N HCl.
The removal capacity of the medium was found to be about
800 mg/L of fluorid e/L of Alumina. Many modifications
of process was suggested by subsequent workers, several
patents based on the use of Aluminum oxide for fluoride
removal were issued 1. Filter alum was used to
regenerate activated alumina bed. The capacity of
alumina to remove fluoride was reported to be
proportional to the amount of filter alum used for
regeneration up to a level of about 0.2kg of alum per
litre of alumina. At this level the fluoride removal
capacity was approximately 500 mg of fluoride per litre
of alumina. Similar studies employing activated alumina
was later conducted by many workers and all these works
confirmed the ability of activated alumina for higher
uptake of fluoride from water. Some researchers have
concluded that removal was the result of ion exchange,
but investigations by others have shown that the process
is one of the adsorption and follows the Langmuir
isotherm model.
Activated
Alumina can be regenerated with HCl, H2SO4, Alum or NaOH.
The use of NaOH needs to be followed by a neutralization
to remove residual NaOH from the bed. Fluoride removal
by activated alumina is strongly pH dependent. Batch
adsorption data14 showed very little removal at pH 11.0
and optimum removal at pH 5.0.Hence raw water pH &
regenerated bed pH need to be ad justed accordingly.
The ability
of activated alumina to remove fluoride depends on other
aspects of the chemistry of water as well. Such factors
as hardness, silica and boron, etc., if present in water
will interfere with fluoride removal and reduce the
efficiency of the system.
The use of
activated alumina in a continuous flow fluidized system
is an economical and efficient method for defluoridating
water supplies15. The process could reduce the fluoride
levels down to 0.1 mg/L. The operational, control and
maintenance problems, mainly clogging of bed, may be
averted in this method.
|
-
Activated alumina
-
Na OH,
-
NaCl
-
2N HCl
-
H2S04
-
Filter
alum
Advantages:
-
It
requires minimum contact time for maximum
defluoridation.
-
Percentage of regeneration is considerably high.
-
There is
very little attritional loss ( to a negligible
extent) during the regeneration at the initial stage
of operation
-
It is
indigenously available and cheap.
-
Defluoridation capacity at neutral pH is
appreciable, although it has greater defluoridation
efficiency at low pH.
-
Its
defluoridation capacity is independent of
temperature.
-
The
effect of other ions present in drinking water, like
chlorides, sulphates and carbonates, over the
defluoridation efficiency of activated alumina is
minimum, eventhough the presence of bicarbonate ions
show considerable influence in the process of
defluoridation.
For cost and
more details - see :
|
|
Bone Char |
-
The
uptake of fluoride onto the surface of bone was one
of the early methods suggested for defluoridation of
water supplies. The process was reportedly one of
the ion exchange in which carbonate radical of the
apatite comprising bone, Ca(PO4)6.CaCO3, was
replaced by fluoride to form an insoluble
fluorapatite. Bone char produced by carbonizing bone
at temperature of 1100-1600ºC had superior qualities
than those of unprocessed bone and hence replaced
bone as defluoridating agent
|
The fluoride
removal capacity of the product is 1000 mg/L
|
|
Contact
Precipitation
|
It is a
technique by which fluoride is removed from the water
through the addition of calcium and phosphate compounds
and then bringing the water in contact with an already
saturated bone charcoal medium.
|
|
|
Degreased
and alkali treated bones |
Degreased
and alkali treated bones are effective in the removal of
fluoride from initial fluoride concentration ranging
from 3.5 mg fluoride/L to 10 mg fluoride/L to less than
0.2 mg fluoride/L
Bone contain
calcium phosphate and has a great affinity for fluoride.
The bone is degreased, dried and powdered. The powder
can be used as a contact bed for removal of fluoride in
water. The exhausted bed is regenerated with sodium
hydroxide solution
|
- |
|
Synthetic
tri-calcium phosphate |
The product
is prepared by reacting phosphoric acid with lime(Bulusu).
The medium is regenerated with 1% NaOH solution followed
by a mild acid rinse
|
It has a
capacity to remove 700 mg fluoride/L |
|
Florex
|
A mixture of
tri-calcium phosphate and Hydroxy -apatite, commercially
called Florex, showed a fluoride removal capacity of 600
mg of fluoride per liter and is regenerated with 1.5%
sodium hydroxide solution. Owing to high attritional
losses, Florex was not successful and the pilot plants
using this material were abandoned
|
- |
|
Activated
Carbon |
Most of the
carbons prepared from different carbonaceous sources
showed fluoride removal capacity after alum
impregnation. High Fluoride removal capacities of
various types of activated carbons had been reported.
Alkali
digested alum impregnated paddy husk carbon was an
efficient defluoridating agent.
Investigations have shown that carbonized saw dust when
quenched in 2% alum solution forms an excellent
defluoridating carbon. The defluoridating process is
stoichiometric and equilibrium is established between
carbon & fluoride. On exhaustion (after continued use)
the carbon can be regenerated by passing 0.2 to 0.5%
alum solutions.
Activated
carbon prepared by other workers from cotton waste,
coffee waste, coconut waste etc., was tried for
defluoridation but all these materials proved to be of
academic interest only
|
Alkali
digested alum impregnated paddy husk carbon
Alkali
digested (1% KOH) & alum soaked (2% alum) carbon removed
320 mg fluoride per kg & showed maximum removal
efficiency at pH 7.0. |
|
Lime |
The
fluorides in waters containing Magnesium, when treated
with lime, are adsorbed on Magnesium hydroxide flocs
enabling fluoride removal12, 25,26.
In this case
the water must be treated to a caustic alkalinity of 30
mg fluoride/L, a pH of 10.5 or above and as such
recarbonation is necessary27.
Magnesia and
calcined magnesite have also been used for fluoride
removal from water and fluoride removal capacity was
reported to be better at high temperature
|
- |
|
Ion Exchange
Resins |
-
Strong
base exchange resins remove fluorides either on
hydroxyl cycle or chloride cycle along with anions.
Since the proportional quantity of fluoride as
compared to other anions is very small, the
effective capacity of such resins works out quite
low. Some inorganic ion exchangers, eg. complex
metal chloride silicates, formed from barium or
ferric chloride with silicic acid, also exchanged
fluoride for chloride.
-
Cation
exchange resins impregnable with alum solution have
been found to act as defluoridating agents. Alum
treated cation exchange resins were used for
defluoridation. ‘Avaram Bark’ based cation exchange
resins, had been reported to work effectively in
removing fluoride from water
-
Polystyrene anion exchange resins in general and
strongly basic quaternary ammonium type resins in
particular are known to remove fluorides from water
along with other anions. The fluoride removals by
various anion exchange resins are given6 in the
table
-
Table 3
indicates that the resins studied yields 20 – 145
bed volume of defluoridated water per cycle.
Subsequent experience showed that these resins lose
their fluoride removal capacity on prolonged use (10
– 15 cycles) and a total replacement becomes
necessary. A layer of white deposits was developed
over the resin beds, and this may be the reason for
this drop in the capacity.
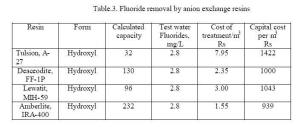
|
Thus the
anion exchange resins were found to be of relatively low
capacity for fluoride removal. The cost of anion resins
is Rs. 20 to 35 per litre. The results indicate that
anion exchange resins are not economical for removing
fluorides from water. Besides, the strong base anion
exchange resins impart a taste to the treated water that
may not be acceptable to the consumers. |
|
Cation
Exchange Resins |
-
Performance of Saw dust carbon (Defluoron–1),
Carbion, Wasoresin – 14 and a polystyrene cation
exchange resin for fluoride removal were compared35
and the results of the study are summarized in the
table.4
|
- |
|
Magnesia |
Investigations were conducted to study the usefulness of
magnesia in fluoride removal. Crystalline magnesium
hydroxide was obtained by reacting a magnesium salt with
milk of lime. The precipitate was filtered, washed and
dried. The dried product was calcined at 1000°C for 3
hours to obtain magnesia. Varying quantities of magnesia
were added to one litre aliquots of test water and
stirred for 30 min using a jar test machine. Fluoride
contents were estimated on one hour settled sample.
A typical
groundwater containing 10 mg/L fluorides, 60 mg/I
hardness, 500 mg/L alkalinity and 7.6 pH was studied
using magnesia (MgO) concentrations of 10 - 1,500 mg/L.
The treated water showed a pH above 9. The average
fluoride concentration in the filtrate was 5.8 mg F/L
where the dose was 1,000 mg/L. The fluoride at 100, 250
and 500 mg/L doses were 9.5, 8.9 and 8.4 mg F/L,
respectively. A dose of 1,500 mg/L magnesia and a
contact period of 3 hr was required to reduce the
fluoride content in the water to 1 mg/L.
The high
initial cost, large concentrations required, alkaline pH
of the treated water and complexity of the preparation
of magnesia are the inhibitive factors to render it
acceptable in the field
|
The study
established that magnesia removed the excess fluorides,
but large doses were necessary. Moreover the pH of the
treated water was beyond 10 and its correction by
acidification or recarbonation was necessary.
All this
adds to the cost and complexity of operations. The acid
requirement can be to the extent of 300 mg/L expressed
in terms of CaCO3/L |
|
Serpentine |
-
Serpentine is a mineral name, which applies to the
material containing one or both of the minerals,
chrysotile and antigorite1. The composition of the
mineral closely corresponds to the formula Mg6Si4O10
(OH). The material is green or yellow and is
available in Andhra Pradesh. To test the capacity of
serpentine to remove fluorides from waters, the
green and yellow varieties were studied for their
defluoridation capacity. Extensive laboratory
investigations were conducted with a view to
popularize the mineral, if found suitable as a
defluoridating medium. A comparative evaluation was
made using green and Yellow varieties of serpentine
and the results are given in the table 5. It is
concluded that cost of defluoridation is prohibitive
with serpentine
|
Materials
like clays, minerals, ion exchange resins, activated
carbons, activated alumina, sulphonated coals and
serpentine were tried for the removal of excess
fluorides from water.
In-situ
chemical treatment with lime, magnesium salts, iron and
aluminum salts were also studied. Those that showed an
encouraging trend on a bench scale were studied in
detail.
These
include ion exchange resins, saw dust carbon, coconut
shell carbon defluoron-1 carbon, magnesia, serpentine
and defluoron-2. Ion exchange resins, saw dust carbon,
defluoron-1, magnesia and serpentine did not prove
useful beyond bench –scale. |
|
Lime stone,
special soils and clay etc |
-
Recently
limestone and heat-treated soil were tried for
fluoride removal. Limestone was used in a two-column
continuous flow system (limestone reactor) to reduce
fluoride concentrations from wastewaters to below
the MCL (Maximum contaminant level) of 4 mg/L.
Calcite was forced to dissolve and fluorite to
precipitate in the first column. The degassing
condition in the second column caused the
precipitation of the calcite dissolved in the first
column, thus returning the treated water to its
approximate initial composition.
-
In
laboratory experiments, the fluoride concentration
of the effluent from all tested feed waters
containing initial fluoride amounts from 10 to 100
mg/L. And a steady state of the system performance
was quickly achieved, For instance, in an experiment
when the input fluoride concentration was 100 mg/L,
effluent concentrations from both columns were below
4 mg/L after only 8 pore volumes had passed. The
proposed reactor has potential application to reduce
concentrations from wastewaters of anionic elements
similar in charge and size to carbonate ion, such as
Selenate and arsenate and cations similar in size
and charge to Ca2+ ,such as Cd2+.
-
Pleistocene soil available locally in Xinzhou, China
was able to remove fluoride from local ground water.
X-ray diffraction analysis revealed that the soil is
composed principally of quartz (50- 60%), Illite
(30-40%), goethite (5-10%) and feldspar (5-10%). A
substantial improvement in both permeability and the
fluoride removal capacity of the soil was achieved
by heating it in a Muffle furnace. A granular
material can then be obtained by crushing the heated
product
-
The
experimental results showed that heating at
400-500ºC has the optimal effect on the enhancement
of the material’s fluoride removal capacity. A
preliminary column experiment showed that 4.0 kg of
400ºC heat-treated soil can treat more than 300L of
5 mg/L fluoride feed water before the effluent
fluoride concentration reaches 1.0 mg/L. Once the
soil’s fluoride-sorption capacity had been reached,
the material could be regenerated in a cost
effective way: rinse the soil first with sodium
carbonate solution, then with dilute HCl and finally
with distilled water twice. After being air-dried
the material is ready for reuse
-
Attempts
were made to use local Kenyan soil derived from
volcanic ash (ex: Ando soils or soils with andic
properties) as a fluoride sorbent37. The ability of
Kenyan Ando soil to adsorb fluoride was determined
experimentally. These results were extended to
possible technical application using a one
dimensional solute transport model. Based on the
result it is concluded that the use of Ando soils
appears to be an economical and efficient method for
defluoridation of drinking water on a small scale in
rural areas of Kenya and other regions along the
Rift zone. Further research is warranted to evaluate
its practical applications and social acceptance.
-
Fluoride
sorption studies were carried out on two clay
minerals, montmorillonite KSF and kaolin, and a
silty clay sediment series (SCSS, used in
earthenware making) 38.The function of fluoride
concentration, clay concentration and pH in
clay-water suspensions was studied. Kaolinite, a
dioctahedral two layered (Silica + alumina)
Silicate(1:2 type),exhibited very little tendency
for Fluoride sorption while montmorillonite,2:1 type
material characterized by Octahedral sheet of
alumina sandwiched between two tetrahedral sheets of
silica, showed significant Fluoride sorption.
The Fluoride sorption on montmorillonite KSF was
found to be greatest at pH 1.9 ± 0.3,the natural pH
of montmorillonite-water suspension. At pH 4.0 ±
0.36, the percentage fluoride sorption on
montmorillonite decreased, followed by an increase
around pH 5-6, after which the percentage decreased
with increasing pH. The applicability of the
Freundlich isotherm was also verified in case of
montmorillonite KSF at low fluoride concentrations.
As a result of fluoride adsorption, increased
release of Fe2+, Cl-, NO3 - ions from
montmorillonite matrix was observed. There was no
effect on SO4 2- or PO4 2- solubility. Fluoride
adsorption on SCSS was also significant and
decreased regularly
with increasing pH.
-
On the
basis of experimental data a plausible mechanism of
fluoride sorption by clay minerals is suggested.
Based on the results of fluoride sorption mentioned
above, a pilot study on defluoridation of water
employing clay (SCSS) as an adsorbent was als o
undertaken which yielded promising results.
-
Removal
of fluoride by adsorption on to low-cost materials
like kaolinite, bentonite, charfines, lignite and
nirmali seeds was investigated

|
-
|
|
Fly Ash |
Retention of
fluoride ion in dynamic experiments on columns packed
with fly ash was studied40 at 20ºC with a series of
aqueous solutions containing 1,5,10,20,50 and 100 mg
fluoride/L/ The flow rate through a 450-g bed was £
2ml/hr.
At the
lowest fluoride concentration(1 mg/L), the fluoride
level in the effluent initially increased and then
gradually decreased down to 0 mg/L after 120 hours.
With higher
fluoride concentrations in the feed solutions, the
fluoride concentration in the effluent steadily
decreased reaching 0 mg/L after 120-168 hours.
|
The fly ash
was an effective sorbent especially at high
concentrations.
|
|
Electro
coagulation
Electrochemical methods |
-
Electro
coagulation process with aluminum bipolar electrodes
was used for defluoridation process41. The influence
of parameters such as inter-electrode distance,
fluoride concentration, temperature and pH of the
solution were investigated and optimized with
synthetic water in batch mode. The optimization
process continued with Oued Souf water (South
Algeria) where the influence of current density and
area/volume ratio on the defluoridation process was
evaluated. The electro coagulation process with
aluminum bipolar electrodes permitted the
defluoridation of Sahara water without adding salts
to the treated water. The aluminum–fluoride weight
ratio attained was 17/1.
-
A
technology of defluoridation through Electrochemical
route has been developed42. The basic principle of
the process is the adsorption of fluoride with
freshly precipitated aluminum hydroxide, which is
generated by the anodic dissolution of aluminum or
its alloys, in an electrochemical cell.
-
Constraints in the above technology: Electricity is
the main raw material and hence wherever electricity
is not available a suitable polar panel can be
installed.
|
The process
utilizes 0.3 to 0.6kwh of electricity per 1000 liters of
water containing 5- 10 mg/L of fluoride.
The anode is
continuously consumed and needs to be replenished. The
process generates sludge at the rate of 80- 100 gm per
1000 liters (on dry basis). |
|
Rare earth
based materials |
New water
treatment processes have been developed for removal of
hazardous anions such as Fluoride, Arsenic, Selenium
species, and phosphate from water using rare earth based
materials which have not been efficiently utilized by
industry in spite of their abundance43. The
state-of-the-art of rare earths in terms of cost, use
and health effects and the environmental problems
associated with hazardous anions in terms of treatment
and toxicity are generally described. Solid lanthanum
and Yttrium ions have been used as adsorbents for
removing hazardous anions. Either lanthanum or Yttrium
ions have been loaded on porous silica or alumina beads
to improve economic and engineering performance; such
rare earth impregnated materials have been successfully
applied to the treatment of synthetic as well as
industrial wastewaters.
A rare earth
metal-based inorganic adsorbent, Cerium- Iron adsorbent
(CFA), was developed and its performance for fluoride
removal from water was evaluated44. The characteristics
of the adsorbent were summarized. Experimental results
show that rare earth metal adsorbents had a relatively
high adsorption capacity and good kinetic property for
fluoride ion removal. The highest capacity was obtained
at pH 3, then it decreased with the increase of pH. The
pH effect however, became inconspicuous when the pH was
over 5.The results show that the adsorption of fluoride
on CFA adsorption follows Freundlich isotherm in the
tested range of fluoride concentrations. The adsorption
capacity could almost be recovered by regenerating it
with 1 molx1-1 NaOH solution
An
adsorbent, which is a mixture of rare earth oxides was
found to adsorb fluoride rapidly and effectively45. The
effect of various parameters such as contact time,
initial concentration, pH and adsorbent dose on
adsorption efficiency was investigated. More than 90% of
the adsorption occurred within the first 5-10 minutes.
Adsorption was found to be dependent on the initial
fluorid concentration and adsorption behavior followed
Langmuir adsorption model. The optimum pH was found to
be about 6.5. The presence of other ions such as nitrate
and sulphate did not affect the adsorption of fluoride
significantly (adsorption efficiency reduced from 85 to
79%) indicating the selective nature of the adsorbent.
The adsorbed fluoride could be easily desorbed by
washing the adsorbent with a pH 12 solutions. This study
clearly shows the applicability of naturally occurring
rare earth oxides as selective adsorbent for fluoride
from solutions
|
- |
|
Tamarind Gel |
The
concentration of fluoride from solution of sodium
fluoride of 10 mg/L could be brought down to 2 mg/L by
the addition of tamarind gel alone and to 0.05 mg/L by
the addition of small quantity of chloride with the
tamarind gel.
|
tamarind gel
small
quantity of chloride |
|
Plant
materials |
The plant
materials such as barks of Moringa olifera and Emblica
officinalis , the roots of Vetiveria zizanoides and the
leaves of Cyanodon tactylon were found to be good
defluoridating agents
|
- |
|
