|
August 13, 2016 from Sci-Hub Website
We overlook a mushrooming threat at our peril, finds Tim Vernimmen
That's probably how most of us think of
fungi. Few people would consider them to be killers. But perhaps we
should.
Then there's our disruption of natural environments, which creates opportunities for fungi to evolve.
Now, some researchers are worried we could be about to reap the spores we've sown:
Neil Gow, a medical mycologist at the University of Aberdeen, UK, was co-organizer of a conference held at London's Royal Society earlier this year to assess the growing fungal threat in areas from animal welfare to food security to ecosystem stability.
He's keen not to overstate the threat to human health - but not to downplay it either.
That is not to say fungi don't kill people.
Even now, about a dozen fungal species (Hidden Killers - Human Fungal Infections) kill in total around 1.5 million people every year.
Fungal disease is a significant contributor to AIDS deaths, for example - and yet the threat is often overlooked.
Fungi comprise a whole kingdom of organisms in their own right, separate from plants and animals, and far less studied.
This hugely diverse group of up to 5 million species includes mushrooms, yeasts, moulds and crop-destroying rusts and smuts.
Most of the time, we happily coexist
even with the killer varieties - you may be inhaling them right now,
or they may be living in or on your body. But occasionally they turn
rogue.
Candida cannot survive without living on us or other animals.
Yet sometimes the unassuming resident
gets a bit too comfortable and multiplies so fast that it causes the
infection commonly known as thrush.
Usually, our white blood cells and other defences do a good job of keeping the fungus under control.
This can be very aggravating - just ask one of the 100 million women worldwide who suffer at least four episodes of vaginal thrush a year.
"More people die from invasive fungal infections than from malaria may cause a local outbreak."
Most people recover without complications, because the fungus seldom thrives in the blood.
But Candida does overcome the defences of hundreds of thousands of people each year to enter their blood - and at least half of them die.
How can this be?
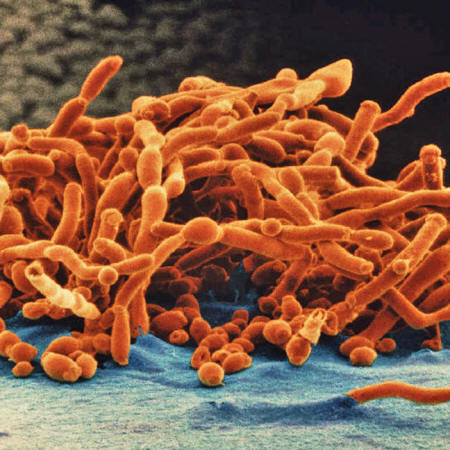
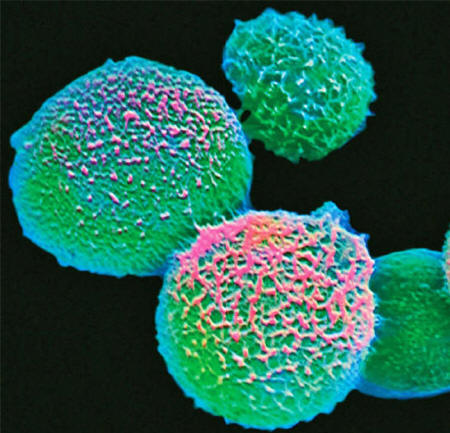
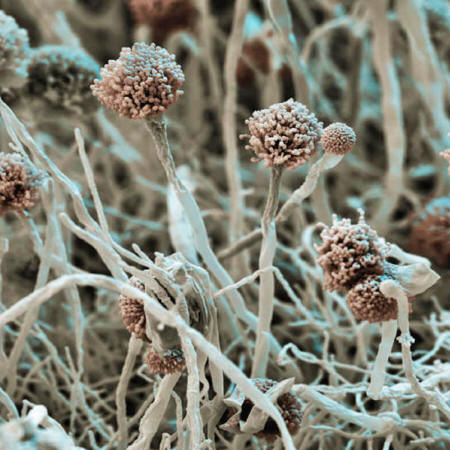
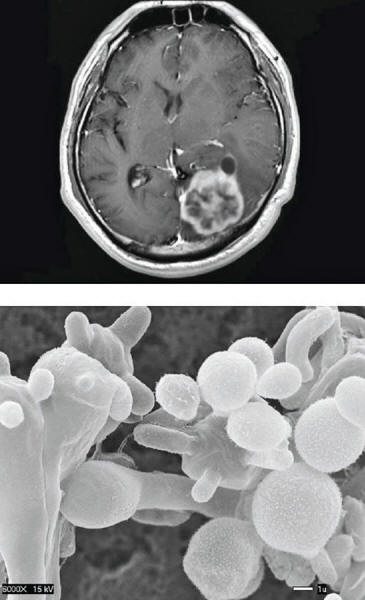
A handful of fungi kill some 1.5 million people each year.
They include Candida,
Cryptococcus and Aspergillus
EYE OF
SCIENCE/SCIENCE PHOTO LIBRARY
E. GUEHO/SCIENCE
PHOTO LIBRARY
Candida uses camouflage and can shed tiny bits of cell wall to avoid being caught.
Even when it does end up inside a white blood cell, it's not game over.
Another potentially deadly fungus,
Cryptococcus, can cause meningitis by lurking in a white blood cell
until it crosses the usually impenetrable blood-brain barrier. It
then forces the cell to eject it.
Yet Cryptococcus has recently achieved something once considered almost impossible:
First discovered in Vancouver, Canada, over a decade ago, a particular strain of Cryptococcus, C. gattii, spread across the Pacific Northwest of the US, killing hundreds along the way.
How does a fungus living on plant matter manage to survive inside a healthy human body?
By accident, argues Robin May.
However, the fungus is preyed upon by amoebas in the soil, and their mode of attack is quite similar to that of white blood cells.
That might give the fungus a head start.
This means it can occasionally thrive inside the body, harming its
host in the process.
The fact that fungi don't depend on us for their survival cuts both ways, though.
Given that there has only been a single outbreak of C. gattii, it's difficult to establish what led to it.
May surmises that the strain had been around for some time, and that a very hot and dry summer may have contributed to its spread.
However, we don't have clear evidence
for this and May notes that the summers of the past decade have all
been fairly wet.
This raises the question of whether other deadly new fungal strains might emerge as climate change takes hold.
That is difficult to answer because the impact on weather patterns is likely to be very variable, says May.
Another concern is that although the warmth of our body protects us from many fungal infections, a warmer world may undo that by helping fungi to adapt.
In any case, Cryptococcus copes just fine with being at 37°C.
Another fungus, called Aspergillus, can live in the heart of compost heaps at temperatures of 60°C.
Aspergillus spores are absolutely everywhere, says Jacques Meis of the Canisius Wilhelmina Hospital in Nijmegen, the Netherlands.
Keeping Aspergillus at bay is a constant challenge.
They are also extremely hardy - the
spores can survive acidity, dehydration, freezing and high heat. No
wonder they're the most common eukaryote on the planet.
The fact that we, too, are eukaryotes makes it difficult to combat fungal pathogens.
So they are often combined with or replaced by another class of antifungal drugs, collectively known as azoles.
One deadly fungal strain has found a way into the human brain.
But lately, Meis has seen an increasing number of patients coming down with a resistant strain right away.
They are now used to prevent fungal growth on crops, produce and flowers, and are an ingredient in many paints and coatings.
Aspergillus isn't the target of these azoles, but it is constantly exposed to them.
Meis doesn't expect companies to stop producing azoles or farmers to stop using them.
Deadly fungal disease is often not viewed with the seriousness it deserves because it mainly affects people that were "on the way out anyway", says medical mycologist David Denning at the University of Manchester, UK.
But that argument is very problematic, he says.
In any case, it isn't true that weakened patients who contract a fungal disease are already bound to die of some other cause, says Denning:
However, this means the number of people vulnerable to fungal disease will go on rising unless we tackle the problem.
Yet that is what is happening, especially in the fight against HIV.
Antiretroviral cocktails are now highly effective, but many people with HIV live in poor countries where it can be difficult for them to take the drugs as prescribed.
A lapse in treatment can cause their white-blood-cell count to drop, at which point any fungus they're exposed to may turn invasive.
There are multiple reasons why the problem is going untackled.
But the task isn't impossible, and cracking it could be a big step towards achieving the UN's target of reducing annual AIDS-related deaths to below 500,000 by 2020.
|