|

by Linda Moulton Howe
2005
from
EarthFiles Website
recovered through
WayBackMachine Website
 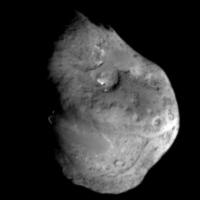
Left: NASA
illustration of Deep Impact's impactor just before hitting Comet
Tempel I on July 4, 2005.
Right: Actual cratered surface of Comet Tempel I ninety seconds
before the impactor smashed
into the fluffy ice at 23,000 mph. Photograph courtesy NASA.

Moment of impact on potato-shaped Comet Tempel I at 10:52 p.m. PDT,
July 3, 2005 / 1:52 a.m. EDT, July 4, 2005. Image by NASA,
ESA, Johns Hopkins University Applied Physics Lab.
August 12, 2005 College Park, Maryland
- This week geologists, chemists,
physicists and planetary scientists from around the world gathered
at the 9th International Asteroids, Comets and Meteors Conference in
Brazil. One of the presentations was by Carey Michael Lisse,
Ph.D., Prof. of Physics at the University of Maryland, and member of
the Deep Impact Science Team. Dr. Lisse is Principal Investigator of
Deep Impact spectral results from the Chandra X-Ray and Spitzer
telescopes.
Before he left for Brazil, Dr. Lisse gave me a preview of what has
been learned so far since the July Fourth impact on Comet Tempel 1.
The impactor hit the comet at 23,000 mph. There was an immediate
flash as the impactor burrowed into the soft, fluffy ice, releasing
hot gases. Essentially, the impactor vaporized, and the comet around
the impactor broke up and vaporized. That happened in about two
seconds and was the first spectra data blast.
Then comet debris flew out. That immediate ejecta did not last very
long either and was the second data blast. Compounding the problems
of those first short-lived spectra bursts, it turns out that the
Deep Impact mothership was not pointing exactly the right way to get
the best spectral results when the impactor slammed into Tempel I.
But back toward Earth, the largest infrared telescope ever launched
into space was watching the Deep Impactor collide with the comet.
The Spitzer Space Telescope was monitoring in a 5 to 40 micron
wavelength range, which was more sensitive than Deep Impact's 1 to 5
micron wavelength range.
Deep Impact was so close, it could only
see a part of the comet's coma, which is the fuzzy haze of glowing
dust that surrounds the comet's hard nucleus. Spitzer could see the
whole coma, the hot gases and the ejecta rushing out from the
explosion for hours and days afterward.
Dr. Lisse told me:
"Of all the data I've seen, Spitzer
is one of the most gorgeous data sets. I think we've got the
first good handle on excavating a comet and finding the dinosaur
bones of the solar system's formation."
The very first data showed hot water. At
least 50% of Comet Tempel I is water ice. The spectra also showed
carbon dioxide and some yet unidentified organic material like
graphite or carbon black. And Spitzer spectral data showed some
surprises such as precursors to the aminos in amino acids. Those are
hydrogen cyanide and methyl cyanide.
Surprisingly, Deep Impact spectra also
show carbonate - think limestone; and polycyclic
aromatic hydrocarbons (PAHs) - think carbon Bucky
Balls and nanotubes.
I asked Dr. Lisse about his conclusions
so far.
Interview:
Carey Michael Lisse, Ph.D., Prof. of Physics, University
of Maryland, College Park, Maryland, and member of the Deep
Impact Science Team and Principal Investigator for the
Chandra X-Ray and Spitzer telescope Deep Impact spectrometer
results:
"What have we concluded so far?
It's still under work, but we've
concluded so far that we see every major rock-forming element
that makes up the Earth in this Comet Tempel I.
EVERY ONE?
Except for iron. The iron is hiding. We're trying to figure that
out. Is it just not easy to see in the infrared? Or is the iron
hiding in an iron oxide or iron sulfide, pyrite or rust.
We think that iron particles or iron sulfide or iron oxide are
all relatively weak and hard to see. They don't make nice
crystals that shine brightly in infrared, unlike silicate such
as sand and other rock-forming elements that you can see really
easy in infrared. You can see alumina or conundrum, which is
sapphire or ruby.
[Editor's Note: Alumina
or Aluminum oxide is a chemical compound of aluminum and
oxygen with chemical formula Al2O3. Commonly referred to as
alumina in the mining, ceramic, and materials science
communities. The gems ruby and sapphire are mostly aluminum
oxide, given their characteristic colors by trace
impurities. Aluminum oxide is an excellent thermal and
electrical insulator. In its crystalline form, called
corundum, its hardness makes it suitable for use as an
abrasive and as a component in cutting tools.]
Looking for Precursors to Life
THERE HAVE BEEN HYPOTHESES OVER THE DECADES ABOUT COMETS
BRINGING LIFE-BEARING PROTEINS AND ORGANIC MOLECULES TO THIS
PLANET AND PERHAPS OTHERS. HAVE YOU FOUND ANYTHING FROM THE DEEP
IMPACT RESEARCH THAT LOOKS LIKE EITHER A PRECURSOR TO LIFE, OR
LIFE ITSELF?
Certainly not something that's life, no amino acid or protein.
We would have yelled and screamed about that if we had seen
anything directly. So nothing that looks like a peptide bond
stretch, which is the building block of amino acids.
But we have found:
1) Hydrogen cyanide (HCN)
and
2) Methyl cyanide, which are precursors of the amino
part of amino acids.
[Editor's Note: HCN =
Hydrogen cyanide is a chemical compound with chemical
formula H-CN. A solution of hydrogen cyanide in water is
called hydrocyanic acid or prussic acid. Pure hydrogen
cyanide is a colorless, very poisonous, and highly volatile
liquid that boils slightly above room temperature at 26 °C
and generates hydrogen cyanide gas. Hydrogen cyanide has a
faint, bitter, almond-like odor and is weakly acidic. HCN
partly converts to the cyanide ion CN in aqueous solution,
resulting in a colorless volatile liquid with the typical
hydrogen cyanide odor. The salts of hydrogen cyanide are
known as cyanides. Used by the chemical industry in
tempering steel, dyeing, explosives, engraving, the
production of acrylic resin plastic, and other organic
chemical products. ]
Ammonia?
We don't have the direct detection
of ammonia or NH2, which would be the direct
precursor. That's one part.
Formic Acid?
The other part would be looking for
an organic acid like formic acid. When you think comets, I want
you to think the very simplest compounds because we don't find
the big, fancy large ones. We don't find proteins, we don't find
a thousand atomic mass polypeptides or polymers or plastics or
anything like that.
We usually find little stuff like
methane, CH4, or carbon dioxide and carbon monoxide
and water, which are very simple, four or five atoms.
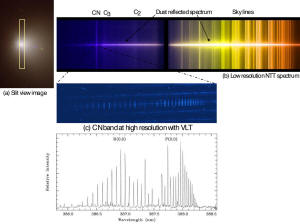
June 2005 spectra
before impact on July 4, 2005:
water, cyclohexane, carbon dioxide, carbon monoxide.
July Fourth
spectra to be released in upcoming journal publications.
HOW MUCH METHANE FROM DEEP IMPACT?
Not clear yet. But we've definitely found:
3) Methanol
4) Carbon Monoxide, CO
5) Carbon Dioxide, CO2
6) Water (Tempel I is about 50% water ice)
7) Ethane (Hydrocarbon consists of two carbons and six
hydrogens; non-soluble in water. It is the simplest
hydrocarbon containing more than one carbon atom.)
These again are all simple
molecules. You can't scream and say, 'Life from them!' But they
are the precursors you need for amino acids and life.
BENZENE?
No, nothing obvious. It's hard to find that. We also see what
are called:
8) 'PAHs' polycyclic
aromatic hydrocarbons.
Think Bucky Balls and
carbon nanotubes and graphite which has been exposed
to hydrogen and water. That could also be catalytic for life or
it could be a component. But there is nothing that shrieks that
there has to be bacteria or life. We don't have that.
9) Carbonate - And we have
also found evidence for carbonate (limestone-like) in
Deep Impact.
[Editor's Note:
Polycyclic aromatic
hydrocarbons (PAHs) are also called polynuclear
aromatic hydrocarbons. They are composed of more than one
aromatic ring. The simplest polycyclic aromatic hydrocarbon
is pentalene.
PAHs of three rings or more have low solubilities in water
and a low vapor pressure. As molecular weight increases,
solubility and vapor pressure decrease. PAHs with two rings
are more soluble in water and more volatile. Because of
these properties, PAHs in the environment are found
primarily in soil and sediment, as opposed to in water or
air. However, PAHs are often found in particles suspended in
water and air.
As molecular weight increases, the carcinogenicity of PAHs
also increases, and acute toxicity decreases. One PAH
compound, benzo[a]pyrene, is notable for being the first
chemical carcinogen to be discovered.
Naphthalene (C10H8), consisting of two
coplanar six-membered rings sharing an edge, is another
aromatic hydrocarbon. By some conventions, it is not a true
PAH, but is referred to as a bicyclic aromatic hydrocarbon.
Its smell is familiar to those who have encountered
mothballs.
PAHs and Origins of Life:
In January 2004, at the 203rd
Meeting of the American Astronomical Society, it was
reported (as cited in Battersby, 2004) that a team led by
A. Witt of the University of Toledo, Ohio studied
ultraviolet light emitted by the Red Rectangle nebula and
found the spectral signatures of PAHs, anthracene and pyrene.
No other such complex molecules had ever before been found
in space.
This discovery was considered
confirmation of a hypothesis that as nebulae of the same
type as the Red Rectangle approach the ends of their lives,
convection currents cause carbon and hydrogen in the
nebulae's core to get caught in stellar winds, and radiate
outward. As they cool, the atoms supposedly bond to each
other in various ways and eventually form particles of a
million or more atoms.
Witt and his team inferred that since they discovered PAHs-which
may have been vital in the formation of early life on Earth
in a nebula, nebulae, by necessity, are where PAHs
originate.
Nanotube:
M. S. Dresselhaus,
Department of Physics and the Department of Electrical
Engineering and Computer Science, Massachusetts Institute of
Technology:
"Conceptually, single-wall
carbon nanotubes (SWCNTs) can be considered to be formed
by the rolling of a single layer of graphite (called a
graphene layer) into a seamless cylinder."
Nanotubes are a proving to be
useful as molecular components for nanotechnology.
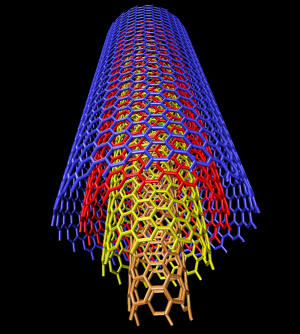
Structure of
a multi-walled nanotube Alain Rochefort,
Center for Research on Computation and its Applications (CERCA).
Bucky Ball:
"C60 is the third
major form of pure carbon. Graphite and diamond are the
other two. C60 is the roundest and most
symmetrical large molecule known to man. Molecules made up
of 60 carbon atoms arranged in a series of interlocking
hexagons and pentagons, forming a structure that looks
similar to a soccer ball. C60 is actually a
"truncated icosahedron", consisting of 12 pentagons and 20
hexagons.
C60 is the only
molecule composed of a single element to form a hollow
spheroid [which gives the potential for filling it, and
using it for novel drug-delivery systems.
"The bucky ball, being the
roundest of round molecules, is also quite resistant to high
speed collisions. In fact, the bucky ball can withstand
slamming into a stainless steel plate at 15,000 mph, merely
bouncing back, unharmed. When compressed to 70 percent of
its original size, the bucky ball becomes more than twice as
hard as its cousin, diamond." ]
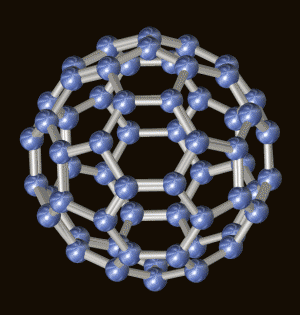
Bucky
Ball, C60 model of molecule. © Dr. Roger C. Wagner,
Dept. of Biological Sciences, University of Delaware.
"We found evidence for PAHs which
have never been seen in comets before. We don't know exactly
what kinds they are. We're pretty sure they are there.
BECAUSE?
We see them around stars. We see them in the interstellar
medium, if you are collecting dust that's silicaceous the
rock-forming elements are there and we know there is a lot of
carbon in the universe. There are more carbon atoms than there
are silicon because carbon is lighter. Carbon has an atomic
weight of 12. Silicon has 28. Basically in the universe, simpler
is more. That's why you have 85% of the universe is hydrogen and
another 7% is helium.
The fewer atoms, the fewer neutrons
and protons to put in, the more stuff you've got because the
universe is still in a relatively simple state. So, you've got a
lot of carbon out there. If we see silicates, we should be
seeing the carbons and carbonates as well.
THAT WOULD IMPLY WHAT?
Carbonate is interesting in that it's like calcium carbonate,
limestone, that you find on the Earth. We do not think there is
any indication for life before I go down that road, or there is
any intimation of that. Carbonates are interesting in that hints
of them were seen once in Comet Halley. They are controversial
because folks will be surprised to see them. Usually you need a
liquid water environment to form them. You have carbon dioxide.
You have water. And you have silicates, then you can form
carbonate.
What's confusing is that comets, as far as we know, were formed
at about 30 to 40 degrees Kelvin above absolute zero. Very cold!
Think ice ball and very little chemistry can happen to them.
On the other hand, you have 5 billion years to make stuff. So
even if you are making things extremely slowly, it's possible
you can make carbonates in a solid state.
Now it's also possible that our chemistry folks will tell us,
that what you did was form the carbonates in the primordial
cloud that condensed into the solar system. That you did your
chemistry before you condensed your comet at 30 to 40 degrees
Kelvin. So this might be another clue as to the recipe to how
things came together.
Precursors to Amino Acids?
WOULD THE FACT THAT YOU HAVE FOUND CARBONATES AND METHANE AND
50% WATER AND CARBONS IN TEMPEL I INDICATE THAT ALL THE
INGREDIENTS ARE THERE FOR MAKING WHAT COULD BE THE CHAINS TOWARD
LIFE WITH PROTEINS AND AMINO ACIDS?
We've got the carbon dioxide, we have the methane, we have the
water. What's missing is ammonia. The amino in the word
'amino acid' is ammonia. But it does not mean ammonia is not
there (on Comet Tempel I). Ammonia has been terribly difficult
to detect very well in comets. We have seen hints of it. We're
pretty sure it is in comets. But it is hard to find.
WHY?
It's just not very active in infrared. To answer your question,
if we get a better handle on the ammonia, the answer is yes.
SINCE AMMONIA DOES NOT SHOW UP ON INFRARED SO EASILY...
Not very well. It would be about 11 microns, which is right in
the middle of a huge silicate, rock-forming element. Hard to
see.
IS IT POSSIBLE THEN THAT YOU COULD HAVE PRECURSORS OF LIFE
MOLECULES ON COMET TEMPEL I OR ANY OF THE OTHER COMETS AND YOU
JUST HAVEN'T BEEN ABLE TO SEE IT?
Yes. It's possible we haven't detected them yet because it's
hard to detect them. Or that they are .001%. But, as we've
probably have learned on Earth, it does not take much. All you
need is a few.
UNTIL YOU CAN GET ACTUAL COMETARY MATTER RETURNED TO THE EARTH
TO A LAB...
In pristine cold condition, I should add that. Star Dust
is going to return dust in January 2006 that has come off the
Comet Wild 2 and been captured briefly at very high temperatures
and velocities in the aerogel. Hopefully we'll be able to keep
the gases as well, but it's not clear if there was any ammonia
or carbon dioxide or water ice that it would not boil off in the
process of capture.
Or hasn't already boiled off once
the dust is returned.
The goal of the
Stardust mission is to return both particle samples from Comet
Wild 2 and from interstellar dust.
By returning these samples to Earth in January 2006 for
analysis, NASA says, "a great deal is expected to be learned
about the
composition of the building blocks of the early solar system and
our neighboring local stellar medium." Image courtesy NASA.
"ALL OF THIS SEEMS RELEVANT TO THE
FUNDAMENTAL QUESTION THAT EVERYBODY WOULD LIKE TO KNOW: IS THERE
OTHER LIFE IN THE UNIVERSE?
And how did we get here?
Do Comets Carry Life Seeding
Molecules?
YES. AND WHY DOES THIS EARTH HAVE SO MUCH WATER AND WHY DOES IT
HAVE LIFE? AND THAT LEADS TO THE QUESTION: HOW LIKELY DO YOU
THINK IT IS THAT WE WILL FIND A COMET THAT HAS ALL THE
INGREDIENTS OF ACTUAL LIFE MOLECULES IN IT?
It's a very good question. Right now, the answer is in the realm
of speculation. I'd like to answer the question in a slightly
different form. I don't mean to sound like I'm managing the
media, but let me back up. We know that we've got an estimate of
the number of stars in our galaxy. It's about 100 billion.
From the Hubble observation of the universe, we think there is
100 billion galaxies. When we look at stars, we think about 10%
of them have planets. So, you take 100 billion stars in our
galaxy and say 10% of them have planets = 10 billion planets, at
least.
Then there is 100 billion galaxies. You wind up saying there are
ten-to-the-21-power planets in the universe. Should I give that
in scientific notation or in billions and billions and
billions..?
(laughing) I KNOW THE DRAKE EQUATION.
I guess what I am trying to say is that we humans might not be
unique, except that we're the only ones that we know."
More
Information:
The Drake Equation:
Bradley Keyes at The
Active Mind describes the Drake Equation developed by
Frank Drake in 1961,
"as a way to focus on the
factors which determine how many intelligent, communicating
civilizations there are in our galaxy.
The Drake Equation is:
N = N* fp ne fl fi fc fL
N* represents the
number of stars in the Milky Way Galaxy. Current
estimates are 100 billion.
fp is the fraction of stars that have planets
around them. Current estimates range from 20% to 50%.
ne is the number of planets per star that are
capable of sustaining life. Current estimates range from
1 to 5.
fl is the fraction of planets in ne where life
evolves. Current estimates range from 100% where life
can evolve, down close to 0%.
fi is the fraction of fl where intelligent life
evolves. Estimates range from 100% down to near 0%.
fc is the fraction of fi that communicates with
rest of universe. Possibly 10% to 20%
fL is fraction of the planet's life during which
the communicating civilizations live. If Earth was
destroyed tomorrow, the answer for our planet's
intelligent life trying to communicate would be only
1/100,000,000th.
When all of these variables are
multiplied together, it equals N, the number of
communicating civilizations in the galaxy."
Where Does All the Water On Earth Come
From?
More Interview with Carey Lisse, Ph.D:
"EARTH IS A PLANET IN WHICH
TWO-THIRDS OF THE SURFACE IS DEEP WATER. DO YOU THINK ALL OF
THIS CAME FROM COMETS?
Some of it could have come from comets. But there is some
evidence from the last two comets we've seen that were very
bright: Comet Hyakutake (1996) and Comet Hale-Bopp(1986). The
isotopic make-up of the Earth's oceans does not look the same as
that of those two comets.
There are arguments from looking at
Hale-Bopp that the oceans can be no more than 30% to 40%
comet-derived.
 
Left: Comet
Hyakatake in late March 1996.
Right: Comet
Hale-Bopp on April 4, 1997
by Astronomiska Observatoriet, Uppsala, Sweden.
There are other arguments that Hale-Bopp
and other comets might not be your typical comets that were in
the plane of the early solar system and we still have to look
for other comets that were. So, if the isotopic data persists in
other comet investigations, it's a very important result even if
now controversial.
Current thinking about the origin of Earth's water is thought to
have come from one of two places, water-bearing rocks or much
later heavy bombardment of comets:
-
It's thought that at the
beginning of Earth's formation, asteroids and comets were
coming in, agglomerating, accreting and melting and all
forming heavy stuff which fell to the center (core) and
formed a giant ball of rock. In the process of doing that,
it lost any original water that came in which probably
boiled off right away. The Earth was a molten lava ball. But
eventually, it cooled off enough that rains came.
-
The water in the rain came from
one of two places: either interior deep rock still had some
water in it. If you take a rock today and you put it in the
oven to roast it, you'd actually pull water out of it, even
from what you thought was a dry rock.
-
So, you either have what we call
'water hydration' in the rocks themselves and that water
came out (on the surface of the Earth). Or, that water came
from what we call an 'Era of Late Bombardment.' We have
evidence that comets came in by showers late after the Earth
was formed and was cooling. We can see evidence for this in
the crater record of the moon and other planets and some of
the asteroids. We know after 100 million years or so, there
were one or two more periods of very heavy bombardment by
bodies. That's why comets might have brought water ice that
eventually became oceans.
IF COMETS WERE INVOLVED IN BRINGING
WATER AND POSSIBLY LIFE-FORMING MOLECULES, WHY WOULD THERE BE A
CONCENTRATION OF WATER AND LIFE ON THE THIRD PLANET FROM THE SUN
AND NOT ON MARS?
It's a good question. Part of it is a Goldilocks and The Three
Bears argument. You have to figure that the sun is so warm.
Think back to Easy Bake Ovens for children. If you are close
enough to a light bulb, you can actually use it to heat and cook
for you.
So, depending upon how close or far
away you are from the Sun, you've reached a certain temperature.
Sort of like coming closer or being father away from a fire. The
Earth is not too close and not too far from the sun. It's just
right for having liquid water and solid water and gaseous water,
which is where a lot of our very rich chemistry happens.
If the Earth were solid water say, you took the Earth and
moved it out to Mars and you kept all its water everything
would become a solid sheet of ice and it would be very hard to
do much chemistry. If there was life, it would move very slowly.
If you moved the Earth to where Venus is, but we didn't have a
greenhouse, we'd be hot. Everything would probably be an ocean
of water. We'd probably have life, but we might have a very
different form of life. Everybody would certainly be swimming!
And it might be too hot for some molecules to be stable.
But we on Earth are just in the
right place where a lot of very subtle and very complex
chemistry can happen.
Why Does Earth Have Life?
It's a very good question. I wish I could answer you. That's one
of the reasons I'm a scientist. One of the things we might be
learning in our life time is that we aren't the only place life
started. It's the reason to go Mars. Now we know there was water
on Mars and we know Mars has CO2 and water in the
(North and South Pole) caps and may have a permafrost there,
frozen underneath the surface.
Did you hear the news report the
other day that a lady is able to grow 2,000-year-old date palms
in Israel from date seeds?

Healthy,
fruit-producing plant has grown from 2000-year-old date palm
seed found in southern Israel Kibbutz Ketura in Arava Desert.
Photograph 2005
by David Blumenfeld, San Francisco Chronicle, June 12, 2005.
These are ancient and are supposed
to be the best dates in the world from the Mediterranean region.
The Judean date palm has died out, but 2,000-year-old seeds are
sprouting. Wherever we look, we see life down in the lake miles
deep underneath the Antarctic ice cap. I know they have gotten
viable bacteria from 3 miles down in the Earth's rock. So, the
question is: if we go to Mars, if there was life at some point
and if things changed so that Mars lost most of its water or
dried out, if the pole of Mars actually wobbles a lot?
Our pole wobbles a little bit and makes massive changes in our
own weather. What if Mars is just like the Earth's a long time
ago, but basically its pole flipped over? Jupiter perturbs it a
lot. What if it suddenly changed its weather and seasons rather
violently? It's possible there was some life at one point and
it's gone to sleep and is still viable.
So I think the jury is out on
whether Mars ever had life. It might not be just the Earth (that
has life).
Not All Comets Are the Same
DOESN'T THAT ALSO MAKE THE POINT THAT IT'S STILL LARGELY UNKNOWN
WHAT COMETS ARE MADE OF AND THEY ARE DIFFERENT FROM COMET TO
COMET? WE'VE ONLY HAD ONE DEEP IMPACT TO ANALYZE SPECTRA UP
CLOSE. THERE COULD BE A WIDE VARIETY OF COMPOSITION IN COMETS?
That's correct. And one of the things we do each time we look at
a comet, we find for example, looking at Tempel I this is
what we thought going into this Deep Impact mission. I'll reach
back two years ago. Comet Tempel I was a boring, average, every
day comet. A little bit different chemically than other short
period comets, but basically it looked like it had been around
the Sun a lot, old, nothing exciting in terms of activity,
quietly boiling off, maybe quietly going toward extinction in
thousands of years, just mildly going around the Sun.
As we got closer and closer to it, we found it had jets
outbursting every few days. We've gotten the up-close images
that will be released soon and there's all kinds of interesting
geology on this body. It's got at least three different terrains
that look very different. It's got young and old craters. We
think it's been cratered for ten million to a hundred million
years. It's got all kinds of structure on it.
We've already seen this from the Star Dust and the DS-1
missions, but this is a fantastically complex body. Tempel I is
nothing like just an ice ball that's been put together 5 billion
years ago and slowly boiling off. It's got a lot of things going
on.
So, to answer your question, I agree the more we study these
comets, the more we might find they have all kinds of different
compositions and behaviors. I think we'll find that Wild-2 is a
very young comet surface and Tempel I is a very old comet
surface, the same thing only older and more evolved because it's
been boiling off and evaporating a lot of the crater features we
saw on Wild-2.
But that jury is out. It's going to
take a little while to figure that out.
Where Do Comets Get All Their Water?
Go back to the universe and its overall composition after the
Big Bang. 85% of the universe is hydrogen. I think another 7
to 10% is helium and the remaining percent is what astronomers
call metals basically, everything heavier than helium.
Since the beginning of the universe, which was 13.8 billion
years ago, stars have been burning the material of the universe
through their fusion reactions and very quickly through the
supernova explosions. So, think about it as the same way you put
gas in your car and you start with a very pure, clear liquid,
you throw all this crap out the back of the car. You throw out
all the complex molecules. Stars have been doing the same thing.
They've been taking hydrogen and
helium and converting them into carbon and nitrogen and oxygen
slowly. Then the supernova explosions go into heavier stuff like
iron, nickel and silica and phosphorous. So, the universe has
slowly been building up the more complicated atoms. Then the
atoms build up more complicated molecules. It's something to
think about our star is a third generation at least sun if it
has lived 4.5 billion years and the universe is 13.7 billion
years old.
The Earth that we're sitting on and these phones we're talking
through and all these atoms and you and I you've heard we're
all star stuff. We've all been through supernova explosions in
the heart of the stars.
And that is true.
How Common Is Water In the Universe?
You've still got a ton of hydrogen in the universe. If you
create an oxygen atom in a supernova explosion or from burning
hydrogen and helium in stars, some of the oxygen is going to get
thrown out into the interstellar medium, the same way it gets
dredged up from the furnace on the inside of the star. If it's a
late type red giant, it's going to encounter other atoms.
One of the things it will make are
rock-forming elements such as silicon oxide and aluminum oxide.
But if it finds a hydrogen, which is more likely than finding
silicon or aluminum atom, it's going to make water. Water is a
very stable molecule. It's very common. You can make water in
late type stars.
Or if in a supernova explosion, you throw out all the oxygen out
into space, the oxygen will cool off and the first thing it's
going to run into is a hydrogen. We know we see water out
between the stars. That's how you make the water. You don't need
to be you can make water even when you're rather cold between
the stars, as long as you have the atoms.
WHEN YOU USE THE WORD WATER YOU MEAN ICE CRYSTALS?
I'm only talking about the molecule H20. I'm not saying it's
water gas, water liquid or water solid.
The other thing that is puzzling, as a chemist I've been
throwing my hands up in astronomy because the things we learn on
the ground when you're sitting there with your test tubes
pouring stuff at room temperature and pressure, none of that
works in space. In space, it's so empty you have a better vacuum
than you've ever seen in the lab. So, when your oxygen atom is
thrown out from a supernova basically shoots out and can take
hundreds of thousands or millions of years, but the first thing
it will see is a hydrogen atom and hook up with it to OH. Then
the next thing it will see will be another hydrogen atom and it
will make H2O.
You don't have a lot around in the vacuum and things can move
very slowly. But they are so large when we talk about the giant
molecular cloud that condensed to form the solar system, it was
probably 5 times the mass of the sun and current solar system.
And it's spread over light years and took millions, or billions
of years, to collapse slowly.
It's the time scales that are hard
for us to think about big, slow ponderous things, massive
things and all very un-dense things all at the same time.
When Will July 4, 2005, Deep Impact
Spectra Be Released to Public?
Spitzer spectra and Deep Impact spectra are brand new and so
exciting we still need to work them over and get them out into
scientific publications. We've put in six to ten years of work
on the project so far, and we want to take more time. So, we're
not releasing them yet.
WHEN WILL THE SPECTRA BE PUBLICLY RELEASED?
In the next few months in science journals. With spectrometers,
you have to be very careful in many ways. It's much harder to
calibrate. For Spitzer, it took about a year or so for the
spectra to get calibrated well. And Deep Impact had only six
months. We're trying to be careful and accurate."
|










